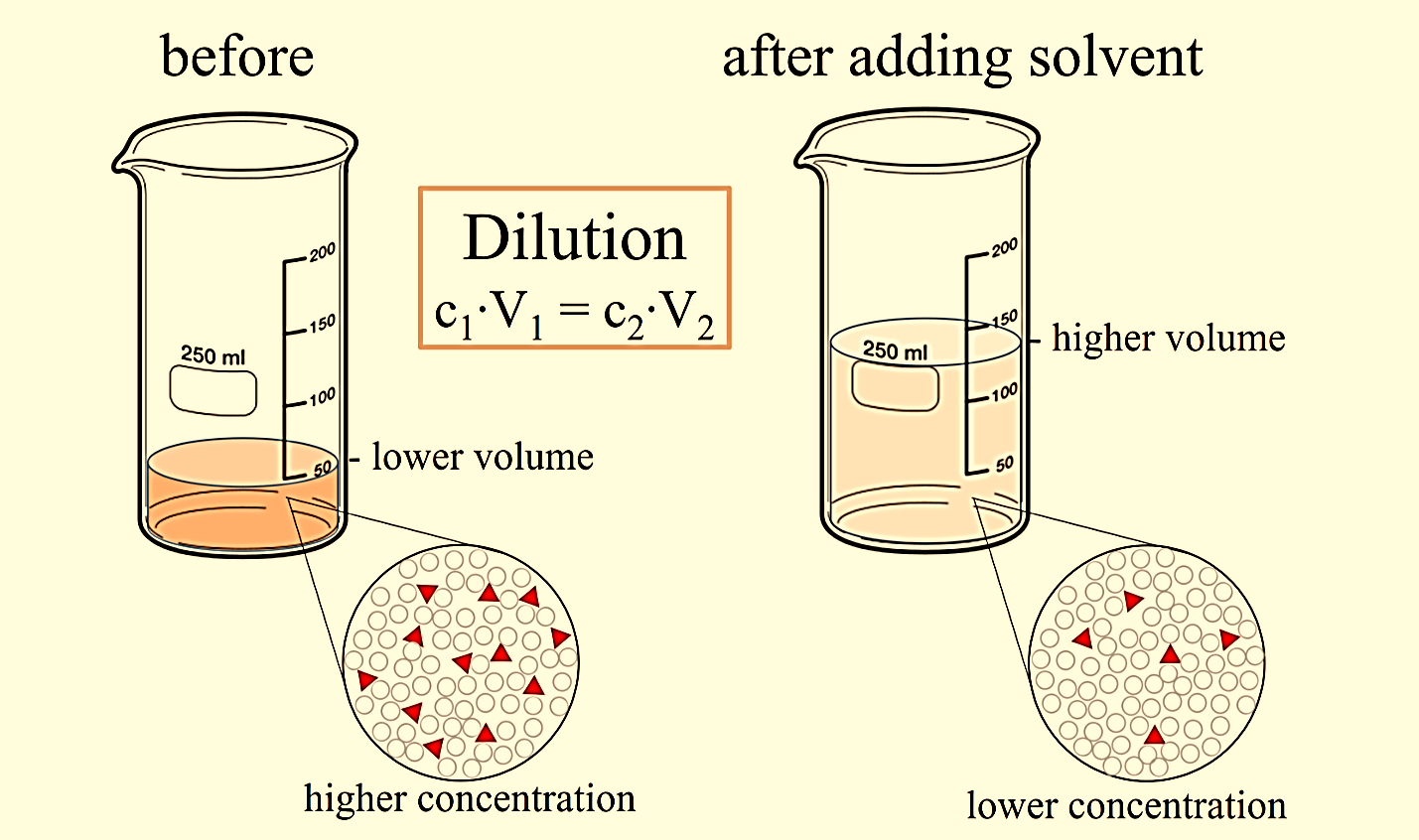
Dilutions Equation . dilution is the process of reducing the concentration of a given solute in its solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. using the dilution equation, we write: Dilution is the process of “lowering the concentration of a solute in a solution. The chemist can do it simply by mixing. state whether the concentration of a solution is directly or indirectly proportional to its volume. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Notice that the volumes need.
from
(1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. using the dilution equation, we write: the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Notice that the volumes need. dilution is the process of reducing the concentration of a given solute in its solution. The chemist can do it simply by mixing. Dilution is the process of “lowering the concentration of a solute in a solution. state whether the concentration of a solution is directly or indirectly proportional to its volume.
Dilutions Equation Dilution is the process of “lowering the concentration of a solute in a solution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. The chemist can do it simply by mixing. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Dilution is the process of “lowering the concentration of a solute in a solution. Notice that the volumes need. using the dilution equation, we write: state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process of reducing the concentration of a given solute in its solution.
From
Dilutions Equation Notice that the volumes need. The chemist can do it simply by mixing. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process of reducing the concentration of a given solute in its solution. using the dilution equation, we write: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x). Dilutions Equation.
From
Dilutions Equation the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Dilution is the process of “lowering the concentration of a solute in a solution. The chemist can do it simply by mixing. dilution is the process of reducing the concentration of a given solute in its solution. state whether. Dilutions Equation.
From
Dilutions Equation The chemist can do it simply by mixing. Dilution is the process of “lowering the concentration of a solute in a solution. dilution is the process of reducing the concentration of a given solute in its solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. the dilution equation is a. Dilutions Equation.
From
Dilutions Equation using the dilution equation, we write: The chemist can do it simply by mixing. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the process of reducing the concentration of a given solute in its solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is. Dilutions Equation.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilutions Equation Dilution is the process of “lowering the concentration of a solute in a solution. dilution is the process of reducing the concentration of a given solute in its solution. using the dilution equation, we write: The chemist can do it simply by mixing. state whether the concentration of a solution is directly or indirectly proportional to its. Dilutions Equation.
From
Dilutions Equation dilution is the process of reducing the concentration of a given solute in its solution. using the dilution equation, we write: state whether the concentration of a solution is directly or indirectly proportional to its volume. The chemist can do it simply by mixing. Notice that the volumes need. the dilution equation is a simple relation. Dilutions Equation.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry Dilutions Equation The chemist can do it simply by mixing. state whether the concentration of a solution is directly or indirectly proportional to its volume. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Notice that the volumes need. Dilution. Dilutions Equation.
From
Dilutions Equation The chemist can do it simply by mixing. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Notice that the volumes need. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. using the dilution equation, we write: Dilution is the process of “lowering the concentration of a. Dilutions Equation.
From
Dilutions Equation (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Dilution is the process of “lowering the concentration of a solute in a solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. using the dilution equation, we write: dilution is the process of reducing the concentration of a given. Dilutions Equation.
From
Dilutions Equation (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the process of reducing the concentration of a given solute in its solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. Notice that the volumes need. the dilution equation is a simple relation between concentrations and volumes. Dilutions Equation.
From
Dilutions Equation Dilution is the process of “lowering the concentration of a solute in a solution. using the dilution equation, we write: Notice that the volumes need. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. The chemist can do it simply by mixing. dilution is the process of reducing. Dilutions Equation.
From
Dilutions Equation (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Dilution is the process of “lowering the concentration of a solute in a solution. Notice that the volumes need. The chemist can do it simply by mixing. dilution is. Dilutions Equation.
From
Dilutions Equation using the dilution equation, we write: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of “lowering the concentration of a solute in a solution. Notice that the volumes need. dilution is the process of reducing. Dilutions Equation.
From
Dilutions Equation dilution is the process of reducing the concentration of a given solute in its solution. using the dilution equation, we write: state whether the concentration of a solution is directly or indirectly proportional to its volume. Notice that the volumes need. the dilution equation is a simple relation between concentrations and volumes of a solution before. Dilutions Equation.
From
Dilutions Equation The chemist can do it simply by mixing. Dilution is the process of “lowering the concentration of a solute in a solution. Notice that the volumes need. using the dilution equation, we write: the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. dilution is the process of reducing. Dilutions Equation.
From
Dilutions Equation state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process of reducing the concentration of a given solute in its solution. Dilution is the process of “lowering the concentration of a solute in a solution. the dilution equation is a simple relation between concentrations and volumes of a. Dilutions Equation.
From
Dilutions Equation Dilution is the process of “lowering the concentration of a solute in a solution. The chemist can do it simply by mixing. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the process of reducing the concentration of a given solute in its solution. state whether the concentration of a solution is directly or. Dilutions Equation.
From www.youtube.com
Dilutions Using M1V1=M2V2 equation YouTube Dilutions Equation using the dilution equation, we write: The chemist can do it simply by mixing. Notice that the volumes need. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process of reducing the concentration of a given solute. Dilutions Equation.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) Dilutions Equation dilution is the process of reducing the concentration of a given solute in its solution. Dilution is the process of “lowering the concentration of a solute in a solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Notice that the volumes need. The chemist can do it simply. Dilutions Equation.
From www.slideserve.com
PPT Chapter 1 The Organization of Matter PowerPoint Presentation Dilutions Equation state whether the concentration of a solution is directly or indirectly proportional to its volume. Notice that the volumes need. The chemist can do it simply by mixing. using the dilution equation, we write: the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Dilution is the process of. Dilutions Equation.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilutions Equation using the dilution equation, we write: the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. state whether the concentration of a solution is directly or indirectly proportional to its volume. The chemist can do it simply by mixing. Notice that the volumes need. (1.50 mol/l) (53.4 ml) =. Dilutions Equation.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilutions Equation Dilution is the process of “lowering the concentration of a solute in a solution. using the dilution equation, we write: The chemist can do it simply by mixing. Notice that the volumes need. state whether the concentration of a solution is directly or indirectly proportional to its volume. the dilution equation is a simple relation between concentrations. Dilutions Equation.
From
Dilutions Equation dilution is the process of reducing the concentration of a given solute in its solution. using the dilution equation, we write: the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. state whether the concentration of a solution is directly or indirectly proportional to its volume. Notice that. Dilutions Equation.
From
Dilutions Equation (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the process of reducing the concentration of a given solute in its solution. Notice that the volumes need. using the dilution equation, we write: The chemist can do it simply by mixing. Dilution is the process of “lowering the concentration of a solute in a. Dilutions Equation.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilutions Equation (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Notice that the volumes need. The chemist can do it simply by mixing. state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of “lowering the concentration of a solute in a solution. using the dilution equation, we. Dilutions Equation.
From
Dilutions Equation state whether the concentration of a solution is directly or indirectly proportional to its volume. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Dilution is the process of “lowering the concentration of a solute in a solution. The chemist can do it simply by mixing. (1.50 mol/l) (53.4. Dilutions Equation.
From
Dilutions Equation state whether the concentration of a solution is directly or indirectly proportional to its volume. Notice that the volumes need. dilution is the process of reducing the concentration of a given solute in its solution. using the dilution equation, we write: Dilution is the process of “lowering the concentration of a solute in a solution. (1.50 mol/l). Dilutions Equation.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilutions Equation Notice that the volumes need. Dilution is the process of “lowering the concentration of a solute in a solution. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. dilution is the process of reducing the concentration of a given solute in its solution. The chemist can do it simply. Dilutions Equation.
From
Dilutions Equation Notice that the volumes need. dilution is the process of reducing the concentration of a given solute in its solution. The chemist can do it simply by mixing. state whether the concentration of a solution is directly or indirectly proportional to its volume. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. using the dilution. Dilutions Equation.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilutions Equation Notice that the volumes need. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the process of reducing the concentration of a given solute in its solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. using the dilution equation, we write: the dilution equation is. Dilutions Equation.
From
Dilutions Equation the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process of reducing the concentration of a given solute in its solution. Dilution is the process of “lowering the concentration of. Dilutions Equation.
From dxlwoimeeco.blob.core.windows.net
Dilution Calculation Formula at Bertha Mohr blog Dilutions Equation The chemist can do it simply by mixing. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of “lowering the concentration of a solute in a solution. the dilution equation is a simple relation between concentrations and. Dilutions Equation.
From
Dilutions Equation Notice that the volumes need. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the process of reducing the concentration of a given solute in its solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of “lowering the concentration of a solute in. Dilutions Equation.
From
Dilutions Equation state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process of reducing the concentration of a given solute in its solution. Notice that the volumes need. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. using the dilution equation, we write: The chemist can do it. Dilutions Equation.
From
Dilutions Equation dilution is the process of reducing the concentration of a given solute in its solution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. using the dilution equation, we write: Notice that the volumes need. state whether the concentration of a solution is directly or indirectly proportional to its volume. the dilution equation is. Dilutions Equation.