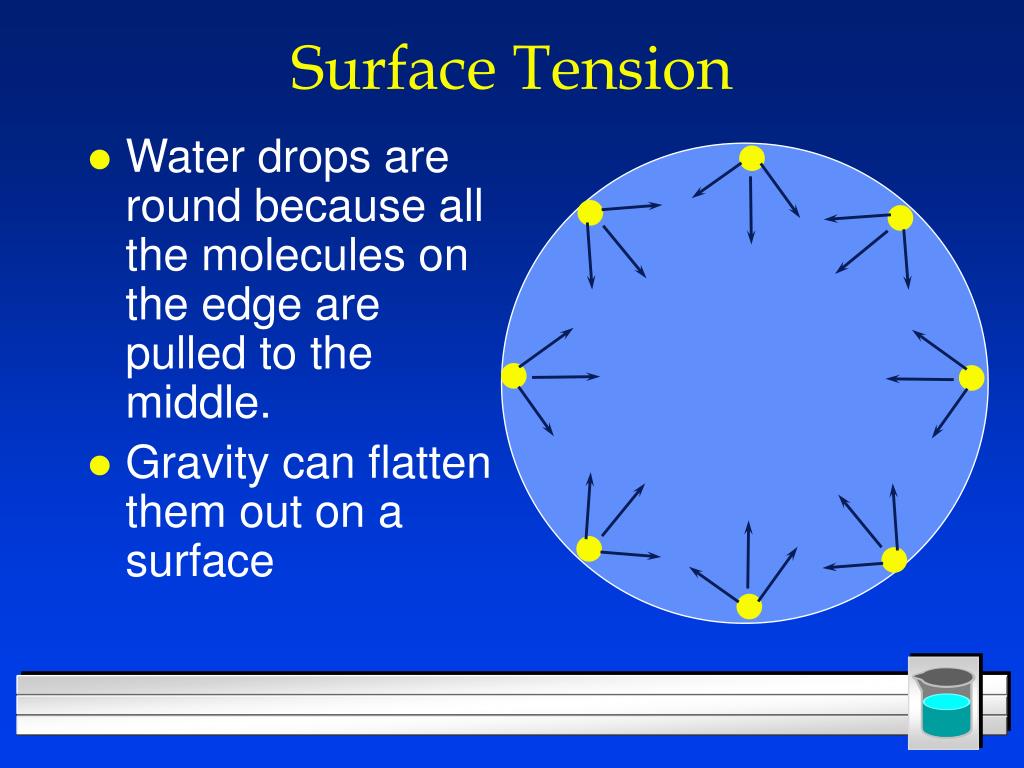
How Is Surface Tension Created Quizlet . The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Water beads up on the surface of a penny because of this property. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Fundamentals of momentum, heat and mass transfer. It is often measured in joules per square meter (\(j/m^2\)). The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. Water droplets take a spherical shape (as pictured) because of. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is.
from www.slideserve.com
Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Fundamentals of momentum, heat and mass transfer. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). Water droplets take a spherical shape (as pictured) because of. It is often measured in joules per square meter (\(j/m^2\)). Water beads up on the surface of a penny because of this property. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount.
PPT Chapter 15 PowerPoint Presentation ID245553
How Is Surface Tension Created Quizlet Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Water droplets take a spherical shape (as pictured) because of. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Water beads up on the surface of a penny because of this property. Fundamentals of momentum, heat and mass transfer. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. It is often measured in joules per square meter (\(j/m^2\)).
From quizlet.com
Use the figure for earlier problem. Find the tension in the Quizlet How Is Surface Tension Created Quizlet Water beads up on the surface of a penny because of this property. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. Fundamentals of momentum, heat and mass transfer. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a. How Is Surface Tension Created Quizlet.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Is Surface Tension Created Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Water beads up on the surface of a penny. How Is Surface Tension Created Quizlet.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it impart YouTube How Is Surface Tension Created Quizlet Water droplets take a spherical shape (as pictured) because of. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the amount of energy required to increase the surface area of a. How Is Surface Tension Created Quizlet.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Illustration of science How Is Surface Tension Created Quizlet Water droplets take a spherical shape (as pictured) because of. Fundamentals of momentum, heat and mass transfer. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. It is often measured in joules per square meter (\(j/m^2\)). The overall effect is that the surface molecules are pulled into the liquid, creating a. How Is Surface Tension Created Quizlet.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences How Is Surface Tension Created Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Water beads up on the surface of a penny because of this property. It is often measured in joules per square meter (\(j/m^2\)). Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount.. How Is Surface Tension Created Quizlet.
From testbook.com
Surface Tension MCQ [Free PDF] Objective Question Answer for Surface Tension Quiz Download Now! How Is Surface Tension Created Quizlet It is often measured in joules per square meter (\(j/m^2\)). Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. Surface tension is a phenomenon in which the surface of a. How Is Surface Tension Created Quizlet.
From www.differencebetween.com
Difference Between Cohesion and Surface Tension Compare the Difference Between Similar Terms How Is Surface Tension Created Quizlet Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Water beads up on the surface of a. How Is Surface Tension Created Quizlet.
From www.purechemistry.org
SURFACE TENSION Purechemistry How Is Surface Tension Created Quizlet Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Water droplets take a spherical shape (as pictured) because of. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. Surface tension is a phenomenon in which the surface of a. How Is Surface Tension Created Quizlet.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 How Is Surface Tension Created Quizlet Water droplets take a spherical shape (as pictured) because of. It is often measured in joules per square meter (\(j/m^2\)). Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is. How Is Surface Tension Created Quizlet.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Is Surface Tension Created Quizlet The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Fundamentals of momentum, heat and mass transfer. Water beads up on the. How Is Surface Tension Created Quizlet.
From www.reddit.com
Determination of Surface Tension Read Chemistry r/ChemistryTeachers How Is Surface Tension Created Quizlet Water beads up on the surface of a penny because of this property. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Fundamentals of momentum, heat and mass transfer. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Water droplets. How Is Surface Tension Created Quizlet.
From testbook.com
Surface Tension & Capillarity with Application, Formula & Examples How Is Surface Tension Created Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Water beads up on the surface of a penny because of this property. Water droplets take a spherical shape (as pictured) because of. Fundamentals of momentum, heat and mass transfer. Surface tension is the amount of energy required to increase the surface area. How Is Surface Tension Created Quizlet.
From studylib.net
surface tension paper1 How Is Surface Tension Created Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. It is often measured in joules per square meter (\(j/m^2\)). Water beads up on the surface of a penny because of this property. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Study with. How Is Surface Tension Created Quizlet.
From www.kiwico.com
Surface Tension Experiment DIY for Beginners KiwiCo How Is Surface Tension Created Quizlet Fundamentals of momentum, heat and mass transfer. Water droplets take a spherical shape (as pictured) because of. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the. How Is Surface Tension Created Quizlet.
From byjus.com
What is surface tension? How Is Surface Tension Created Quizlet Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see. How Is Surface Tension Created Quizlet.
From www.youtube.com
Surface Tension Experiment by Ansa YouTube How Is Surface Tension Created Quizlet Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Water droplets take a spherical shape (as pictured) because of. It is often measured in joules per square meter (\(j/m^2\)). The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like. How Is Surface Tension Created Quizlet.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Consequences Chemistry Notes How Is Surface Tension Created Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Water beads up on the surface of a penny because of this property. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Water droplets take a spherical shape (as pictured) because of. Study with quizlet and. How Is Surface Tension Created Quizlet.
From zanzibarvle.org
Course Surface Tension How Is Surface Tension Created Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. Water beads up on. How Is Surface Tension Created Quizlet.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Consequences Chemistry Notes How Is Surface Tension Created Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Water beads up on the surface of a penny because of this property. It is often measured in joules per square meter (\(j/m^2\)). Study with. How Is Surface Tension Created Quizlet.
From www.diplomageeks.com
Define capilirity, viscosity, coefficient of viscosity and surface tension with examples How Is Surface Tension Created Quizlet It is often measured in joules per square meter (\(j/m^2\)). Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Water beads up on the surface of a penny because of this property. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that. How Is Surface Tension Created Quizlet.
From www.pinterest.com
Surface tension on a penny science Surface tension, Science, Tension How Is Surface Tension Created Quizlet The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). It is often measured in joules per square meter (\(j/m^2\)). Water droplets take a spherical shape (as pictured) because of. The surface tension of a liquid is a measure of the elastic. How Is Surface Tension Created Quizlet.
From www.studypool.com
SOLUTION Surface tension theory Studypool How Is Surface Tension Created Quizlet Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Water beads up on the surface of a penny because of this property. Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. It is often measured in joules per square meter (\(j/m^2\)).. How Is Surface Tension Created Quizlet.
From knowfhysics.blogspot.com
What is surface tension? How Is Surface Tension Created Quizlet Fundamentals of momentum, heat and mass transfer. Water droplets take a spherical shape (as pictured) because of. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. It is often measured in joules per square meter (\(j/m^2\)). Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has. How Is Surface Tension Created Quizlet.
From www.youtube.com
What causes surface tension quizlet? YouTube How Is Surface Tension Created Quizlet It is often measured in joules per square meter (\(j/m^2\)). The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Water droplets take a spherical shape (as pictured) because of. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Cohesive forces between molecules cause the surface. How Is Surface Tension Created Quizlet.
From quizlet.com
A tension member constructed of an L 4 x 4 x \frac{1}{2} i Quizlet How Is Surface Tension Created Quizlet Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). It is often measured in joules per square meter (\(j/m^2\)). Surface tension. How Is Surface Tension Created Quizlet.
From tfiglobalnews.com
The Relation Between Surface Tension and Surface Energy TFIGlobal How Is Surface Tension Created Quizlet Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Fundamentals of momentum, heat and mass transfer. Water droplets take a spherical shape (as pictured) because of. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Water beads up on the surface of. How Is Surface Tension Created Quizlet.
From www.pinterest.com
Fun science experiment for kids using toothpicks on water to learn about surface tension. 5th How Is Surface Tension Created Quizlet The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Fundamentals of momentum, heat and mass transfer. Water droplets take a spherical shape (as pictured) because. How Is Surface Tension Created Quizlet.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Is Surface Tension Created Quizlet Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). It is often measured in joules per square meter (\(j/m^2\)). Surface tension is. How Is Surface Tension Created Quizlet.
From mirjamglessmer.com
Trying to understand surface tension Adventures in Oceanography and Teaching How Is Surface Tension Created Quizlet Study with quizlet and memorize flashcards containing terms like surface tension, surface tensions result from, water has a higher. Water beads up on the surface of a penny because of this property. It is often measured in joules per square meter (\(j/m^2\)). Surface tension is the amount of energy required to increase the surface area of a liquid by a. How Is Surface Tension Created Quizlet.
From www.pw.live
Surface Tension How Is Surface Tension Created Quizlet Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Fundamentals of momentum, heat and mass transfer. It is often measured in joules per square meter (\(j/m^2\)). The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Water droplets take a spherical shape (as pictured) because of.. How Is Surface Tension Created Quizlet.
From fsz.ifas.ufl.edu
Surface Tension How Is Surface Tension Created Quizlet Surface tension is a phenomenon in which the surface of a liquid, where the liquid is. Water beads up on the surface of a penny because of this property. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Cohesive forces between molecules cause the surface of a liquid to. How Is Surface Tension Created Quizlet.
From www.youtube.com
Surface Tension experiment with card and water glass 🍷 YouTube How Is Surface Tension Created Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Water droplets take a spherical shape (as pictured) because of. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). Surface tension is the amount. How Is Surface Tension Created Quizlet.
From www.esaral.com
Mind Maps for Fluid Surface Tension Revision Class 11, JEE, NEET How Is Surface Tension Created Quizlet Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. Water droplets take a spherical shape (as pictured) because of. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). Study with. How Is Surface Tension Created Quizlet.
From civilmint.com
What is Surface Tension Definition of Surface Tension, Examples and Test How Is Surface Tension Created Quizlet Fundamentals of momentum, heat and mass transfer. Surface tension is the amount of energy required to increase the surface area of a liquid by a certain amount. The surface tension of a liquid is a measure of the elastic force within the liquid's surface. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is.. How Is Surface Tension Created Quizlet.
From gosciencegirls.com
7 Surface Tension Experiments To Try With Kids How Is Surface Tension Created Quizlet The surface tension of a liquid is a measure of the elastic force within the liquid's surface. The overall effect is that the surface molecules are pulled into the liquid, creating a surface that is tightened like a film (see a in the figure below). Surface tension is the amount of energy required to increase the surface area of a. How Is Surface Tension Created Quizlet.