Zinc Bromide Electrolysis Half Equations . The half reaction for the oxidation reaction, omitting phase labels, is as follows: For example, in the electrolysis of copper chloride,. Pb 2+ ions gain electrons at the cathode and become pb atoms. Molten lead bromide, pbbr 2 (l), is an electrolyte. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. For molten ionic substances, there are some rules to writing half equations. At the positive electrode (anode) bromine gas is produced by the discharge of. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: An ionic equation shows the overall reaction by combining half equations. Pb 2+ ions gain electrons at the cathode and become pb atoms. Molten lead bromide, pbbr 2 (l), is an electrolyte. The lead (ii) ions are reduced to lead atoms by gaining electrons. In general, the metal is produced at the cathode.
from www.numerade.com
For molten ionic substances, there are some rules to writing half equations. For example, in the electrolysis of copper chloride,. Molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb atoms. Pb 2+ ions gain electrons at the cathode and become pb atoms. The lead (ii) ions are reduced to lead atoms by gaining electrons. The half reaction for the oxidation reaction, omitting phase labels, is as follows: In general, the metal is produced at the cathode. An ionic equation shows the overall reaction by combining half equations. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is:
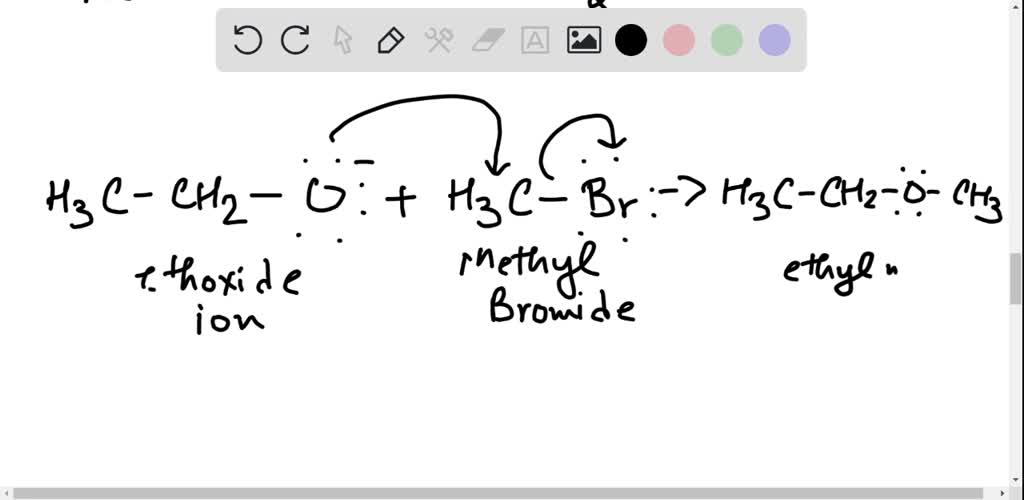
SOLVEDWhen isopropyl bromide is treated with sodium ethoxide in ethanol, propylene and ethyl
Zinc Bromide Electrolysis Half Equations The half reaction for the oxidation reaction, omitting phase labels, is as follows: The lead (ii) ions are reduced to lead atoms by gaining electrons. In general, the metal is produced at the cathode. Pb 2+ ions gain electrons at the cathode and become pb atoms. An ionic equation shows the overall reaction by combining half equations. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: For example, in the electrolysis of copper chloride,. Molten lead bromide, pbbr 2 (l), is an electrolyte. The half reaction for the oxidation reaction, omitting phase labels, is as follows: Pb 2+ ions gain electrons at the cathode and become pb atoms. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. Molten lead bromide, pbbr 2 (l), is an electrolyte. At the positive electrode (anode) bromine gas is produced by the discharge of. For molten ionic substances, there are some rules to writing half equations.
From byjus.com
Mechanism of Hoffman bromide degradation reaction Zinc Bromide Electrolysis Half Equations Molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb atoms. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: Pb 2+ ions gain electrons at the cathode and become pb atoms. Molten lead bromide, pbbr 2 (l), is an electrolyte. \[\ce{zn. Zinc Bromide Electrolysis Half Equations.
From www.numerade.com
SOLVEDWhen isopropyl bromide is treated with sodium ethoxide in ethanol, propylene and ethyl Zinc Bromide Electrolysis Half Equations For example, in the electrolysis of copper chloride,. At the positive electrode (anode) bromine gas is produced by the discharge of. The half reaction for the oxidation reaction, omitting phase labels, is as follows: \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. For molten ionic substances, there are some rules to writing half equations. Molten lead bromide, pbbr 2 (l),. Zinc Bromide Electrolysis Half Equations.
From www.pw.live
Zinc Bromide Formula, Structure And Properties Zinc Bromide Electrolysis Half Equations Molten lead bromide, pbbr 2 (l), is an electrolyte. For molten ionic substances, there are some rules to writing half equations. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: In general, the metal is produced at the cathode. Molten lead bromide, pbbr 2. Zinc Bromide Electrolysis Half Equations.
From www.numerade.com
SOLVEDWrite equations for the halfreactions that occur in the electrolysis of a mixture of Zinc Bromide Electrolysis Half Equations Pb 2+ ions gain electrons at the cathode and become pb atoms. At the positive electrode (anode) bromine gas is produced by the discharge of. For example, in the electrolysis of copper chloride,. The half reaction for the oxidation reaction, omitting phase labels, is as follows: An ionic equation shows the overall reaction by combining half equations. Molten lead bromide,. Zinc Bromide Electrolysis Half Equations.
From www.slideserve.com
PPT What is electrolysis? PowerPoint Presentation, free download ID4564902 Zinc Bromide Electrolysis Half Equations The half reaction for the oxidation reaction, omitting phase labels, is as follows: In general, the metal is produced at the cathode. An ionic equation shows the overall reaction by combining half equations. Pb 2+ ions gain electrons at the cathode and become pb atoms. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. Pb 2+ ions gain electrons at the. Zinc Bromide Electrolysis Half Equations.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Zinc Bromide Electrolysis Half Equations In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: In general, the metal is produced at the cathode. Pb 2+ ions gain electrons at the cathode and become pb atoms. The lead (ii) ions are reduced to lead atoms by gaining electrons. The half reaction for the oxidation reaction, omitting phase labels,. Zinc Bromide Electrolysis Half Equations.
From www.youtube.com
Ethene + Hydrogen Bromide (Reaction and Mechanism) YouTube Zinc Bromide Electrolysis Half Equations The half reaction for the oxidation reaction, omitting phase labels, is as follows: \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. Pb 2+ ions gain electrons at the cathode and become pb atoms. The lead (ii) ions are reduced to lead atoms by gaining electrons. Pb 2+ ions gain electrons at the cathode and become pb atoms. Molten lead bromide,. Zinc Bromide Electrolysis Half Equations.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Zinc Bromide Electrolysis Half Equations \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. Molten lead bromide, pbbr 2 (l), is an electrolyte. For molten ionic substances, there are some rules to writing half equations. In general, the metal is produced at the cathode. For example, in the electrolysis of copper chloride,. Pb 2+ ions gain electrons at the cathode and become pb atoms. Pb 2+. Zinc Bromide Electrolysis Half Equations.
From byjus.com
Write the molecular formulae for the compound Copper II Bromide Zinc Bromide Electrolysis Half Equations An ionic equation shows the overall reaction by combining half equations. The half reaction for the oxidation reaction, omitting phase labels, is as follows: For molten ionic substances, there are some rules to writing half equations. In general, the metal is produced at the cathode. Pb 2+ ions gain electrons at the cathode and become pb atoms. \[\ce{zn → zn^{2+}. Zinc Bromide Electrolysis Half Equations.
From wordwall.net
Electrolysis half equations Lead Bromide Labelled diagram Zinc Bromide Electrolysis Half Equations Pb 2+ ions gain electrons at the cathode and become pb atoms. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: The lead (ii) ions are reduced to lead atoms by gaining electrons. For molten ionic substances, there are some rules to writing half equations. At the positive electrode (anode) bromine gas. Zinc Bromide Electrolysis Half Equations.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 Zinc Bromide Electrolysis Half Equations In general, the metal is produced at the cathode. At the positive electrode (anode) bromine gas is produced by the discharge of. For molten ionic substances, there are some rules to writing half equations. An ionic equation shows the overall reaction by combining half equations. Molten lead bromide, pbbr 2 (l), is an electrolyte. For example, in the electrolysis of. Zinc Bromide Electrolysis Half Equations.
From en.ppt-online.org
Electrolysis online presentation Zinc Bromide Electrolysis Half Equations For example, in the electrolysis of copper chloride,. Pb 2+ ions gain electrons at the cathode and become pb atoms. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: The half reaction for the oxidation reaction, omitting phase labels, is as follows: \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. In general,. Zinc Bromide Electrolysis Half Equations.
From sielc.com
Vinyl bromide SIELC Technologies Zinc Bromide Electrolysis Half Equations At the positive electrode (anode) bromine gas is produced by the discharge of. Pb 2+ ions gain electrons at the cathode and become pb atoms. An ionic equation shows the overall reaction by combining half equations. The lead (ii) ions are reduced to lead atoms by gaining electrons. For molten ionic substances, there are some rules to writing half equations.. Zinc Bromide Electrolysis Half Equations.
From www.macsenlab.com
Pinaverium Bromide API 53251948 Manufacturer & Supplier Zinc Bromide Electrolysis Half Equations Molten lead bromide, pbbr 2 (l), is an electrolyte. Molten lead bromide, pbbr 2 (l), is an electrolyte. The lead (ii) ions are reduced to lead atoms by gaining electrons. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. Pb 2+ ions gain electrons at the cathode and become pb atoms. At the positive electrode (anode) bromine gas is produced by. Zinc Bromide Electrolysis Half Equations.
From www.gauthmath.com
Solved A teacher demonstrates the electrolysis of molten lead bromide. Bromine is produced at Zinc Bromide Electrolysis Half Equations \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. An ionic equation shows the overall reaction by combining half equations. For example, in the electrolysis of copper chloride,. The half reaction for the oxidation reaction, omitting phase labels, is as follows: Molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb. Zinc Bromide Electrolysis Half Equations.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector (Royalty Free) 1977753731 Zinc Bromide Electrolysis Half Equations The lead (ii) ions are reduced to lead atoms by gaining electrons. The half reaction for the oxidation reaction, omitting phase labels, is as follows: At the positive electrode (anode) bromine gas is produced by the discharge of. For example, in the electrolysis of copper chloride,. Pb 2+ ions gain electrons at the cathode and become pb atoms. In the. Zinc Bromide Electrolysis Half Equations.
From www.numerade.com
SOLVED Aqueous solutions of zinc bromide and sodium phosphate combine to produce a precipitate Zinc Bromide Electrolysis Half Equations At the positive electrode (anode) bromine gas is produced by the discharge of. The half reaction for the oxidation reaction, omitting phase labels, is as follows: Pb 2+ ions gain electrons at the cathode and become pb atoms. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: In general, the metal is. Zinc Bromide Electrolysis Half Equations.
From testbook.com
Lithium Bromide Learn Structure, Properties, Preparation & Uses. Zinc Bromide Electrolysis Half Equations Molten lead bromide, pbbr 2 (l), is an electrolyte. The lead (ii) ions are reduced to lead atoms by gaining electrons. In general, the metal is produced at the cathode. An ionic equation shows the overall reaction by combining half equations. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. Pb 2+ ions gain electrons at the cathode and become pb. Zinc Bromide Electrolysis Half Equations.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion Zinc Bromide Electrolysis Half Equations Molten lead bromide, pbbr 2 (l), is an electrolyte. For molten ionic substances, there are some rules to writing half equations. An ionic equation shows the overall reaction by combining half equations. The half reaction for the oxidation reaction, omitting phase labels, is as follows: \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. The lead (ii) ions are reduced to. Zinc Bromide Electrolysis Half Equations.
From www.researchgate.net
Top Chemical structures of CPEs and salts tetraethylammonium bromide... Download Scientific Zinc Bromide Electrolysis Half Equations In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: Pb 2+ ions gain electrons at the cathode and become pb atoms. The half reaction for the oxidation reaction, omitting phase labels, is as follows: For example, in the electrolysis of copper chloride,. Molten lead bromide, pbbr 2 (l), is an electrolyte. Molten. Zinc Bromide Electrolysis Half Equations.
From byjus.com
Briefly explain the process of electrolysis of molten Lead Bromide Zinc Bromide Electrolysis Half Equations Molten lead bromide, pbbr 2 (l), is an electrolyte. The half reaction for the oxidation reaction, omitting phase labels, is as follows: In general, the metal is produced at the cathode. Pb 2+ ions gain electrons at the cathode and become pb atoms. At the positive electrode (anode) bromine gas is produced by the discharge of. Molten lead bromide, pbbr. Zinc Bromide Electrolysis Half Equations.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Zinc Bromide Electrolysis Half Equations Molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb atoms. For example, in the electrolysis of copper chloride,. An ionic equation shows the overall reaction by combining half equations. For molten ionic substances, there are some rules to writing half equations. Molten lead bromide, pbbr 2 (l), is an. Zinc Bromide Electrolysis Half Equations.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Illustration Illustration of Zinc Bromide Electrolysis Half Equations In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: For molten ionic substances, there are some rules to writing half equations. Pb 2+ ions gain electrons at the cathode and become pb atoms. In general, the metal is produced at the cathode. Molten lead bromide, pbbr 2 (l), is an electrolyte. The. Zinc Bromide Electrolysis Half Equations.
From www.numerade.com
SOLVED The compound zinc bromide, ZnBr2 is soluble in water. Write the net ionic equation for Zinc Bromide Electrolysis Half Equations The lead (ii) ions are reduced to lead atoms by gaining electrons. Pb 2+ ions gain electrons at the cathode and become pb atoms. Molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb atoms. At the positive electrode (anode) bromine gas is produced by the discharge of. \[\ce{zn →. Zinc Bromide Electrolysis Half Equations.
From www.chemistrylearner.com
Sodium Bromate Facts, Formula, Properties, Uses, Safety Data Zinc Bromide Electrolysis Half Equations The half reaction for the oxidation reaction, omitting phase labels, is as follows: An ionic equation shows the overall reaction by combining half equations. For molten ionic substances, there are some rules to writing half equations. At the positive electrode (anode) bromine gas is produced by the discharge of. Pb 2+ ions gain electrons at the cathode and become pb. Zinc Bromide Electrolysis Half Equations.
From www.bigstockphoto.com
Test Bromides Iodides Image & Photo (Free Trial) Bigstock Zinc Bromide Electrolysis Half Equations Pb 2+ ions gain electrons at the cathode and become pb atoms. In general, the metal is produced at the cathode. Molten lead bromide, pbbr 2 (l), is an electrolyte. For example, in the electrolysis of copper chloride,. For molten ionic substances, there are some rules to writing half equations. Molten lead bromide, pbbr 2 (l), is an electrolyte. Pb. Zinc Bromide Electrolysis Half Equations.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Zinc Bromide Electrolysis Half Equations For molten ionic substances, there are some rules to writing half equations. Molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb atoms. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: The half reaction for the oxidation reaction, omitting phase labels,. Zinc Bromide Electrolysis Half Equations.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Zinc Bromide Electrolysis Half Equations The half reaction for the oxidation reaction, omitting phase labels, is as follows: In general, the metal is produced at the cathode. Molten lead bromide, pbbr 2 (l), is an electrolyte. Molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb atoms. An ionic equation shows the overall reaction by. Zinc Bromide Electrolysis Half Equations.
From www.numerade.com
SOLVED Write equations for the halfreactions that occur in the electrolysis of molten Zinc Bromide Electrolysis Half Equations At the positive electrode (anode) bromine gas is produced by the discharge of. Molten lead bromide, pbbr 2 (l), is an electrolyte. Molten lead bromide, pbbr 2 (l), is an electrolyte. The half reaction for the oxidation reaction, omitting phase labels, is as follows: In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode). Zinc Bromide Electrolysis Half Equations.
From byjus.com
Phenyl magnesium bromide reacts with methanol to give Zinc Bromide Electrolysis Half Equations For molten ionic substances, there are some rules to writing half equations. The half reaction for the oxidation reaction, omitting phase labels, is as follows: Pb 2+ ions gain electrons at the cathode and become pb atoms. \[\ce{zn → zn^{2+} + 2e^{−}} \nonumber \] this. Pb 2+ ions gain electrons at the cathode and become pb atoms. In the electrolysis. Zinc Bromide Electrolysis Half Equations.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Illustration of stuff, alcohol Zinc Bromide Electrolysis Half Equations Pb 2+ ions gain electrons at the cathode and become pb atoms. The half reaction for the oxidation reaction, omitting phase labels, is as follows: At the positive electrode (anode) bromine gas is produced by the discharge of. For molten ionic substances, there are some rules to writing half equations. For example, in the electrolysis of copper chloride,. The lead. Zinc Bromide Electrolysis Half Equations.
From www.numerade.com
SOLVED Solid zinc (Zn) and aqueous aluminum bromide (AlBr3) are produced by the reaction of Zinc Bromide Electrolysis Half Equations In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: An ionic equation shows the overall reaction by combining half equations. For example, in the electrolysis of copper chloride,. At the positive electrode (anode) bromine gas is produced by the discharge of. The half reaction for the oxidation reaction, omitting phase labels, is. Zinc Bromide Electrolysis Half Equations.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Zinc Bromide Electrolysis Half Equations Molten lead bromide, pbbr 2 (l), is an electrolyte. An ionic equation shows the overall reaction by combining half equations. Pb 2+ ions gain electrons at the cathode and become pb atoms. In general, the metal is produced at the cathode. For molten ionic substances, there are some rules to writing half equations. Molten lead bromide, pbbr 2 (l), is. Zinc Bromide Electrolysis Half Equations.
From mavericksrpatterson.blogspot.com
Chemical Formula of Bromide MavericksrPatterson Zinc Bromide Electrolysis Half Equations Pb 2+ ions gain electrons at the cathode and become pb atoms. At the positive electrode (anode) bromine gas is produced by the discharge of. In the electrolysis of molten lead (ii) bromide the half equation at the negative electrode (cathode) is: In general, the metal is produced at the cathode. The lead (ii) ions are reduced to lead atoms. Zinc Bromide Electrolysis Half Equations.
From www.slideserve.com
PPT 6.2 Redox Reactions Changes at the electrodes PowerPoint Presentation ID4564892 Zinc Bromide Electrolysis Half Equations For molten ionic substances, there are some rules to writing half equations. In general, the metal is produced at the cathode. Molten lead bromide, pbbr 2 (l), is an electrolyte. An ionic equation shows the overall reaction by combining half equations. Molten lead bromide, pbbr 2 (l), is an electrolyte. The lead (ii) ions are reduced to lead atoms by. Zinc Bromide Electrolysis Half Equations.