Hydrate Worksheet Answers . 120.1 / 30.0 = 4. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. An anhydrate is the substance that remains after the water is removed from a hydrate. Ch2o x 4 = c4h8o4. • the process of removing water from a hydrate, usually through applied heat. 3) name the following compounds: What percentage of water is found in cuso 4 * 5h 2 o? Find the fonnula and the chemical name of the hydrate. 159.607 g this is the answer to (b) remember, the anhydrate does. What percentage of water is found is na. When a hydrate is heated the water molecules are. This is the answer to (a) 3) determine the molar mass of the anhydrate: Composition of hydrates worksheet (red book) 1.
from www.numerade.com
Composition of hydrates worksheet (red book) 1. What percentage of water is found in cuso 4 * 5h 2 o? 120.1 / 30.0 = 4. Find the fonnula and the chemical name of the hydrate. 159.607 g this is the answer to (b) remember, the anhydrate does. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. What percentage of water is found is na. Ch2o x 4 = c4h8o4. • the process of removing water from a hydrate, usually through applied heat. When a hydrate is heated the water molecules are.
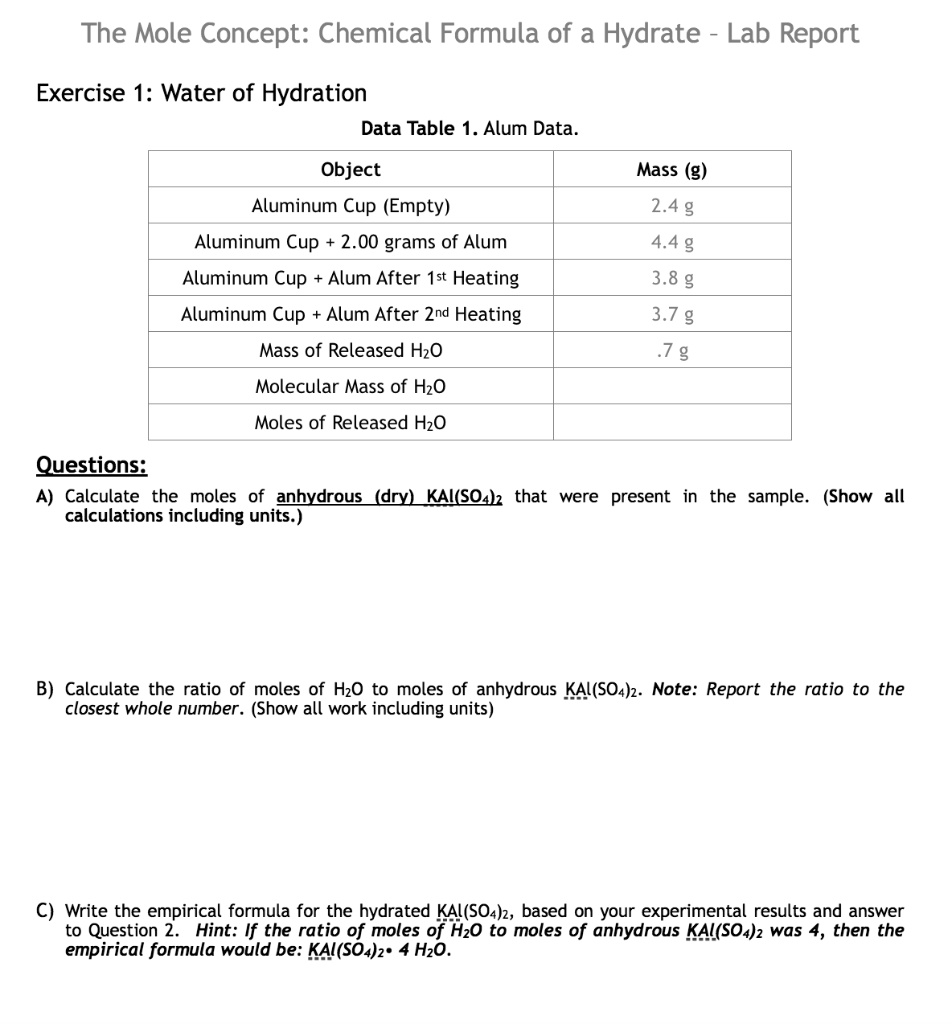
The Mole Concept Chemical Formula of a Hydrate Lab Report Exercise 1
Hydrate Worksheet Answers This is the answer to (a) 3) determine the molar mass of the anhydrate: What percentage of water is found in cuso 4 * 5h 2 o? 120.1 / 30.0 = 4. 3) name the following compounds: Find the fonnula and the chemical name of the hydrate. When a hydrate is heated the water molecules are. This is the answer to (a) 3) determine the molar mass of the anhydrate: What percentage of water is found is na. An anhydrate is the substance that remains after the water is removed from a hydrate. Composition of hydrates worksheet (red book) 1. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. 159.607 g this is the answer to (b) remember, the anhydrate does. • the process of removing water from a hydrate, usually through applied heat. Ch2o x 4 = c4h8o4.
From www.studocu.com
Hydrate Worksheet asdf HYDRATE AND EMPIRICAL FORMULA QUESTIONS Hydrate Worksheet Answers What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. • the process of removing water from a hydrate, usually through applied heat. An anhydrate is the substance that remains after the water is removed from a hydrate. What percentage of water is found is na. 3) name the following. Hydrate Worksheet Answers.
From worksheets.clipart-library.com
Composition of Hydrates Worksheet Red Book Exercises Chemistry Hydrate Worksheet Answers 159.607 g this is the answer to (b) remember, the anhydrate does. This is the answer to (a) 3) determine the molar mass of the anhydrate: 3) name the following compounds: An anhydrate is the substance that remains after the water is removed from a hydrate. Find the fonnula and the chemical name of the hydrate. Composition of hydrates worksheet. Hydrate Worksheet Answers.
From dragadalro.weebly.com
!FREE! Naminghydratesworksheetanswers Hydrate Worksheet Answers 120.1 / 30.0 = 4. 3) name the following compounds: Composition of hydrates worksheet (red book) 1. What percentage of water is found is na. An anhydrate is the substance that remains after the water is removed from a hydrate. Ch2o x 4 = c4h8o4. When a hydrate is heated the water molecules are. What is the molecular formula of. Hydrate Worksheet Answers.
From lolipopppppp.blogspot.com
Hydrates Worksheet Answers Hydrate Worksheet Hydrate Worksheet Name Hydrate Worksheet Answers Find the fonnula and the chemical name of the hydrate. When a hydrate is heated the water molecules are. 120.1 / 30.0 = 4. 3) name the following compounds: Ch2o x 4 = c4h8o4. • the process of removing water from a hydrate, usually through applied heat. What percentage of water is found is na. Composition of hydrates worksheet (red. Hydrate Worksheet Answers.
From materialmediasimeon77.z13.web.core.windows.net
Naming Hydrates Worksheet Hydrate Worksheet Answers An anhydrate is the substance that remains after the water is removed from a hydrate. Find the fonnula and the chemical name of the hydrate. 120.1 / 30.0 = 4. What percentage of water is found is na. 3) name the following compounds: • the process of removing water from a hydrate, usually through applied heat. 159.607 g this is. Hydrate Worksheet Answers.
From lessonlistwilkins.z21.web.core.windows.net
Acid Naming Worksheet And Key Hydrate Worksheet Answers Ch2o x 4 = c4h8o4. Find the fonnula and the chemical name of the hydrate. An anhydrate is the substance that remains after the water is removed from a hydrate. 120.1 / 30.0 = 4. • the process of removing water from a hydrate, usually through applied heat. This is the answer to (a) 3) determine the molar mass of. Hydrate Worksheet Answers.
From printablelibhaloed.z21.web.core.windows.net
Percent Composition Of Hydrates Lab Answers Hydrate Worksheet Answers What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. 159.607 g this is the answer to (b) remember, the anhydrate does. What percentage of water is found is na. Find the fonnula and the chemical name of the hydrate. 3) name the following compounds: • the process of removing. Hydrate Worksheet Answers.
From www.chegg.com
Solved Name or Formula Part 4 Hydrates (optionalcheck with Hydrate Worksheet Answers Find the fonnula and the chemical name of the hydrate. An anhydrate is the substance that remains after the water is removed from a hydrate. What percentage of water is found in cuso 4 * 5h 2 o? This is the answer to (a) 3) determine the molar mass of the anhydrate: What percentage of water is found is na.. Hydrate Worksheet Answers.
From materialfullyeates.z21.web.core.windows.net
Composition Of Hydrates Worksheets Hydrate Worksheet Answers 120.1 / 30.0 = 4. Ch2o x 4 = c4h8o4. Find the fonnula and the chemical name of the hydrate. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. • the process of removing water from a hydrate, usually through applied heat. Composition of hydrates worksheet (red book) 1.. Hydrate Worksheet Answers.
From www.chegg.com
Solved Determining the Formula of a Hydrate Chem Worksheet Hydrate Worksheet Answers What percentage of water is found in cuso 4 * 5h 2 o? • the process of removing water from a hydrate, usually through applied heat. 3) name the following compounds: 120.1 / 30.0 = 4. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. 159.607 g this is. Hydrate Worksheet Answers.
From studyzonezucchinis.z21.web.core.windows.net
Composition Of Hydrates Worksheets Hydrate Worksheet Answers What percentage of water is found in cuso 4 * 5h 2 o? 3) name the following compounds: What percentage of water is found is na. Ch2o x 4 = c4h8o4. An anhydrate is the substance that remains after the water is removed from a hydrate. Find the fonnula and the chemical name of the hydrate. • the process of. Hydrate Worksheet Answers.
From learningmeier.z21.web.core.windows.net
Composition Of Hydrates Worksheet Hydrate Worksheet Answers 159.607 g this is the answer to (b) remember, the anhydrate does. An anhydrate is the substance that remains after the water is removed from a hydrate. When a hydrate is heated the water molecules are. Find the fonnula and the chemical name of the hydrate. This is the answer to (a) 3) determine the molar mass of the anhydrate:. Hydrate Worksheet Answers.
From www.numerade.com
The Mole Concept Chemical Formula of a Hydrate Lab Report Exercise 1 Hydrate Worksheet Answers An anhydrate is the substance that remains after the water is removed from a hydrate. This is the answer to (a) 3) determine the molar mass of the anhydrate: Find the fonnula and the chemical name of the hydrate. 3) name the following compounds: 159.607 g this is the answer to (b) remember, the anhydrate does. What percentage of water. Hydrate Worksheet Answers.
From lolipopppppp.blogspot.com
Hydrates Worksheet Answers Hydrate Worksheet Hydrate Worksheet Name Hydrate Worksheet Answers • the process of removing water from a hydrate, usually through applied heat. What percentage of water is found is na. Composition of hydrates worksheet (red book) 1. What percentage of water is found in cuso 4 * 5h 2 o? 3) name the following compounds: Find the fonnula and the chemical name of the hydrate. 120.1 / 30.0 =. Hydrate Worksheet Answers.
From www.transtutors.com
(Get Answer) Worksheet IV Extra Nomenclature Exercises Hydrates Salts Hydrate Worksheet Answers What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. 120.1 / 30.0 = 4. Composition of hydrates worksheet (red book) 1. Find the fonnula and the chemical name of the hydrate. 159.607 g this is the answer to (b) remember, the anhydrate does. What percentage of water is found. Hydrate Worksheet Answers.
From www.chemmybear.com
AP Chemistry Page Hydrate Worksheet Answers What percentage of water is found in cuso 4 * 5h 2 o? 3) name the following compounds: Find the fonnula and the chemical name of the hydrate. What percentage of water is found is na. Ch2o x 4 = c4h8o4. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass. Hydrate Worksheet Answers.
From www.compoundworksheets.com
Nomenclature 21 Ionic Compounds And Hydrates Worksheet Answers Hydrate Worksheet Answers • the process of removing water from a hydrate, usually through applied heat. When a hydrate is heated the water molecules are. An anhydrate is the substance that remains after the water is removed from a hydrate. This is the answer to (a) 3) determine the molar mass of the anhydrate: Composition of hydrates worksheet (red book) 1. What percentage. Hydrate Worksheet Answers.
From ataglance.randstad.com
Composition Of Hydrates Worksheet Printable Calendars AT A GLANCE Hydrate Worksheet Answers Find the fonnula and the chemical name of the hydrate. Composition of hydrates worksheet (red book) 1. 159.607 g this is the answer to (b) remember, the anhydrate does. What percentage of water is found is na. This is the answer to (a) 3) determine the molar mass of the anhydrate: 3) name the following compounds: When a hydrate is. Hydrate Worksheet Answers.
From correo.muycomputer.com
Composition Of Hydrates Worksheet Printable Kids Entertainment Hydrate Worksheet Answers 3) name the following compounds: Ch2o x 4 = c4h8o4. Find the fonnula and the chemical name of the hydrate. What percentage of water is found is na. 120.1 / 30.0 = 4. 159.607 g this is the answer to (b) remember, the anhydrate does. • the process of removing water from a hydrate, usually through applied heat. What is. Hydrate Worksheet Answers.
From lolipopppppp.blogspot.com
Hydrates Worksheet Answers Hydrate Worksheet Hydrate Worksheet Name Hydrate Worksheet Answers 120.1 / 30.0 = 4. 3) name the following compounds: Find the fonnula and the chemical name of the hydrate. This is the answer to (a) 3) determine the molar mass of the anhydrate: What percentage of water is found is na. Composition of hydrates worksheet (red book) 1. 159.607 g this is the answer to (b) remember, the anhydrate. Hydrate Worksheet Answers.
From circuitwiringmarco.z19.web.core.windows.net
Hydrates Worksheet Answers Hydrate Worksheet Answers Composition of hydrates worksheet (red book) 1. • the process of removing water from a hydrate, usually through applied heat. This is the answer to (a) 3) determine the molar mass of the anhydrate: 120.1 / 30.0 = 4. 159.607 g this is the answer to (b) remember, the anhydrate does. An anhydrate is the substance that remains after the. Hydrate Worksheet Answers.
From worksheets.clipart-library.com
Hydrates Percent Composition Worksheet Answers Exercises Hydrate Worksheet Answers • the process of removing water from a hydrate, usually through applied heat. What percentage of water is found is na. Composition of hydrates worksheet (red book) 1. An anhydrate is the substance that remains after the water is removed from a hydrate. 120.1 / 30.0 = 4. 3) name the following compounds: Find the fonnula and the chemical name. Hydrate Worksheet Answers.
From goodimg.co
️Ionic Hydrate Worksheet Free Download Goodimg.co Hydrate Worksheet Answers 159.607 g this is the answer to (b) remember, the anhydrate does. • the process of removing water from a hydrate, usually through applied heat. When a hydrate is heated the water molecules are. What percentage of water is found in cuso 4 * 5h 2 o? Composition of hydrates worksheet (red book) 1. 120.1 / 30.0 = 4. What. Hydrate Worksheet Answers.
From www.numerade.com
Determining the Formula of a Hydrate Name Chem Worksheet 116 Hydrates Hydrate Worksheet Answers 3) name the following compounds: 120.1 / 30.0 = 4. Ch2o x 4 = c4h8o4. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. • the process of removing water from a hydrate, usually through applied heat. This is the answer to (a) 3) determine the molar mass of. Hydrate Worksheet Answers.
From www.vrogue.co
Composition Of Hydrates Worksheet Printable Word Sear vrogue.co Hydrate Worksheet Answers 159.607 g this is the answer to (b) remember, the anhydrate does. What percentage of water is found in cuso 4 * 5h 2 o? What percentage of water is found is na. This is the answer to (a) 3) determine the molar mass of the anhydrate: • the process of removing water from a hydrate, usually through applied heat.. Hydrate Worksheet Answers.
From worksheetlistas.z19.web.core.windows.net
Determine The Formula Of A Hydrate Worksheet Hydrate Worksheet Answers 3) name the following compounds: • the process of removing water from a hydrate, usually through applied heat. Ch2o x 4 = c4h8o4. This is the answer to (a) 3) determine the molar mass of the anhydrate: What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. What percentage of. Hydrate Worksheet Answers.
From lessonlistsulcalize.z22.web.core.windows.net
Composition Of Hydrates Worksheets Hydrate Worksheet Answers Find the fonnula and the chemical name of the hydrate. What percentage of water is found is na. • the process of removing water from a hydrate, usually through applied heat. 120.1 / 30.0 = 4. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. An anhydrate is the. Hydrate Worksheet Answers.
From study.com
Quiz & Worksheet Determining the Chemical Formula of Hydrates from Hydrate Worksheet Answers 120.1 / 30.0 = 4. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar mass of. This is the answer to (a) 3) determine the molar mass of the anhydrate: • the process of removing water from a hydrate, usually through applied heat. Find the fonnula and the chemical name of. Hydrate Worksheet Answers.
From answerlistjurgen.z19.web.core.windows.net
Hydrates Worksheets Hydrate Worksheet Answers When a hydrate is heated the water molecules are. An anhydrate is the substance that remains after the water is removed from a hydrate. • the process of removing water from a hydrate, usually through applied heat. 120.1 / 30.0 = 4. What is the molecular formula of the molecule that has an empirical formula of ch2cl and a molar. Hydrate Worksheet Answers.
From www.vrogue.co
Determining The Formula Of A Hydrate Chem Worksheet 1 vrogue.co Hydrate Worksheet Answers An anhydrate is the substance that remains after the water is removed from a hydrate. 159.607 g this is the answer to (b) remember, the anhydrate does. Ch2o x 4 = c4h8o4. 3) name the following compounds: Composition of hydrates worksheet (red book) 1. What percentage of water is found is na. This is the answer to (a) 3) determine. Hydrate Worksheet Answers.
From www.chegg.com
Solved Determining the Formula of a Hydrate Name auta Hydrate Worksheet Answers Find the fonnula and the chemical name of the hydrate. An anhydrate is the substance that remains after the water is removed from a hydrate. 3) name the following compounds: 120.1 / 30.0 = 4. This is the answer to (a) 3) determine the molar mass of the anhydrate: Ch2o x 4 = c4h8o4. What is the molecular formula of. Hydrate Worksheet Answers.
From www.chegg.com
Solved Worksheet IV Extra Nomenclature Exercises Hydrates Hydrate Worksheet Answers 120.1 / 30.0 = 4. 159.607 g this is the answer to (b) remember, the anhydrate does. Ch2o x 4 = c4h8o4. When a hydrate is heated the water molecules are. This is the answer to (a) 3) determine the molar mass of the anhydrate: Find the fonnula and the chemical name of the hydrate. Composition of hydrates worksheet (red. Hydrate Worksheet Answers.
From gambr.co
️Naming Hydrates Worksheet 6 Answers Free Download Gambr.co Hydrate Worksheet Answers Find the fonnula and the chemical name of the hydrate. 159.607 g this is the answer to (b) remember, the anhydrate does. An anhydrate is the substance that remains after the water is removed from a hydrate. Composition of hydrates worksheet (red book) 1. • the process of removing water from a hydrate, usually through applied heat. What percentage of. Hydrate Worksheet Answers.
From lolipopppppp.blogspot.com
Hydrates Worksheet Answers Hydrate Worksheet Hydrate Worksheet Name Hydrate Worksheet Answers Find the fonnula and the chemical name of the hydrate. When a hydrate is heated the water molecules are. 3) name the following compounds: What percentage of water is found in cuso 4 * 5h 2 o? Composition of hydrates worksheet (red book) 1. • the process of removing water from a hydrate, usually through applied heat. Ch2o x 4. Hydrate Worksheet Answers.
From www.chegg.com
EXPERIMENT 6 REPORT FORNM Formula of a Hydrate and Hydrate Worksheet Answers Find the fonnula and the chemical name of the hydrate. This is the answer to (a) 3) determine the molar mass of the anhydrate: An anhydrate is the substance that remains after the water is removed from a hydrate. Composition of hydrates worksheet (red book) 1. 120.1 / 30.0 = 4. What percentage of water is found in cuso 4. Hydrate Worksheet Answers.