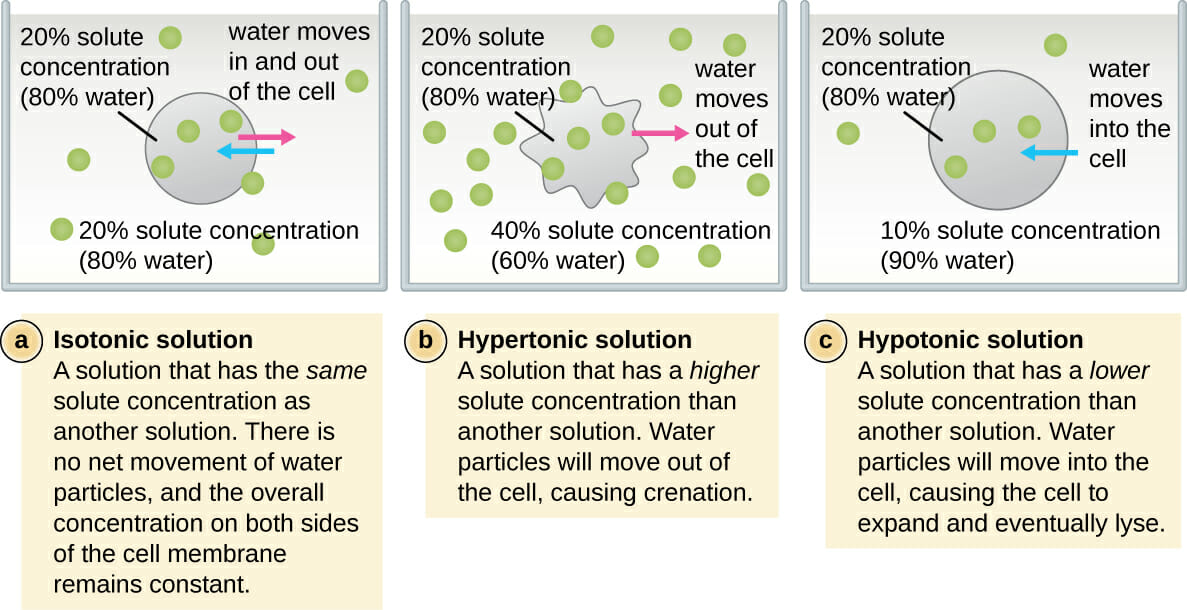
Osmolality Isotonic Solution . Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; If the red cells stay the same size,. This term describes the same concentration of solutes across two areas and is called a. An isotonic solution is the goal of both osmosis and diffusion. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154. The reflection coefficient of a semipermeable membrane describes how well. The osmolality of a solution describes how many particles are dissolved in the solution. The variance can be significant for concentrated solutions.
from biologydictionary.net
The variance can be significant for concentrated solutions. The reflection coefficient of a semipermeable membrane describes how well. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. An isotonic solution is the goal of both osmosis and diffusion. 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. The osmolality of a solution describes how many particles are dissolved in the solution. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. If the red cells stay the same size,.
Isotonic vs. Hypotonic vs. Hypertonic Solution Biology
Osmolality Isotonic Solution Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. An isotonic solution is the goal of both osmosis and diffusion. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. If the red cells stay the same size,. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. The osmolality of a solution describes how many particles are dissolved in the solution. This term describes the same concentration of solutes across two areas and is called a. The reflection coefficient of a semipermeable membrane describes how well. The variance can be significant for concentrated solutions.
From www.researchgate.net
Volume changes of HEK293 cells in response to solutions of varying Osmolality Isotonic Solution Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; This term describes the same concentration of solutes across two areas and is called a. The osmolality of a solution describes how many particles are dissolved in the solution. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry. Osmolality Isotonic Solution.
From www.slideserve.com
PPT Fundamentals of Nursing II 2 nd year PowerPoint Presentation Osmolality Isotonic Solution If the red cells stay the same size,. An isotonic solution is the goal of both osmosis and diffusion. The variance can be significant for concentrated solutions. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; The osmolality of a solution describes how many particles are dissolved in the solution. Osmolality is a colligative. Osmolality Isotonic Solution.
From www.slideserve.com
PPT ENTERAL NUTRITION PowerPoint Presentation ID225635 Osmolality Isotonic Solution 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. This term describes the same concentration. Osmolality Isotonic Solution.
From www.semanticscholar.org
Table 4 from Types of Hyponatremia by Plasma Osmolality Isotonic Osmolality Isotonic Solution This term describes the same concentration of solutes across two areas and is called a. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. An isotonic solution is the goal of both osmosis and diffusion. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. The reflection coefficient of a semipermeable membrane describes. Osmolality Isotonic Solution.
From wtcs.pressbooks.pub
15.3 Intravenous Solutions Nursing Fundamentals 2e Osmolality Isotonic Solution If the red cells stay the same size,. The osmolality of a solution describes how many particles are dissolved in the solution. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. This term describes the same concentration of solutes across two areas and is called a. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and. Osmolality Isotonic Solution.
From www.vrogue.co
Difference Between Isotonic Hypotonic And Hypertonic vrogue.co Osmolality Isotonic Solution An isotonic solution is the goal of both osmosis and diffusion. The reflection coefficient of a semipermeable membrane describes how well. If the red cells stay the same size,. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry. Osmolality Isotonic Solution.
From www.researchgate.net
Differential effects of isotonic and hypotonic solutions on membrane Osmolality Isotonic Solution Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; An isotonic solution is the goal of both osmosis and diffusion. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. The reflection coefficient of a semipermeable membrane describes how well. The. Osmolality Isotonic Solution.
From pediaa.com
Difference Between Osmolarity and Tonicity Osmolality Isotonic Solution Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. The osmolality of a solution describes how many particles are dissolved in the solution. This term describes the same concentration of solutes across two areas and is called a. The variance. Osmolality Isotonic Solution.
From www.athleticinsight.com
Isotonic Solution Definition, How it Works, Examples, and Benefits Osmolality Isotonic Solution If the red cells stay the same size,. An isotonic solution is the goal of both osmosis and diffusion. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. The reflection coefficient of a semipermeable membrane describes how well. 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l),. Osmolality Isotonic Solution.
From pharmacyscope.com
Buffered Isotonic Solutions Pharmacy Scope Osmolality Isotonic Solution Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. The osmolality of a solution describes how many particles are dissolved in the solution. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; The variance can be significant. Osmolality Isotonic Solution.
From www.news-medical.net
Determining the Osmolality of Isotonic Drinks Osmolality Isotonic Solution The variance can be significant for concentrated solutions. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. The reflection coefficient of a semipermeable membrane describes how well. An isotonic solution is the goal of both osmosis and diffusion. If the red cells stay. Osmolality Isotonic Solution.
From www.studypool.com
SOLUTION Isotonic solutions iv fluids cheat sheet nurseslabs Studypool Osmolality Isotonic Solution An isotonic solution is the goal of both osmosis and diffusion. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; The reflection coefficient of a semipermeable membrane describes how well. If the red cells stay the same size,. Osmolality is a colligative property of solutions that depends on. Osmolality Isotonic Solution.
From www.slideserve.com
PPT Intravenous Therapy IV Infusion Preparations Fluid and Osmolality Isotonic Solution Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; The variance can be significant for concentrated solutions. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. The reflection coefficient of a semipermeable membrane describes how well. Osmolality. Osmolality Isotonic Solution.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube Osmolality Isotonic Solution Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. The osmolality of a solution describes how many particles are dissolved in the solution. 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium. Osmolality Isotonic Solution.
From www.slideserve.com
PPT TONICITY, OSMOTICITY, OSMOLALITY & OSMOLARITY PowerPoint Osmolality Isotonic Solution The osmolality of a solution describes how many particles are dissolved in the solution. If the red cells stay the same size,. The variance can be significant for concentrated solutions. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. This. Osmolality Isotonic Solution.
From www.pinterest.com
iv solutions chart Google Search Nursing school survival, Nurse Osmolality Isotonic Solution An isotonic solution is the goal of both osmosis and diffusion. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. If the red cells stay the same size,. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term. Osmolality Isotonic Solution.
From www.sciencefacts.net
Isotonic Solution Definition, Meaning, Examples & Diagram Osmolality Isotonic Solution Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. If the red cells stay the same size,. The osmolality of. Osmolality Isotonic Solution.
From slideplayer.com
Anesthesia For Intracranial Aneurysms ppt download Osmolality Isotonic Solution Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. An isotonic solution is the goal of both osmosis and diffusion. The variance can be significant for concentrated solutions. Osmolarity and osmolality are units of solute concentration that are often used. Osmolality Isotonic Solution.
From www.slideserve.com
PPT LPNC PowerPoint Presentation, free download ID317603 Osmolality Isotonic Solution 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154. The reflection coefficient of a semipermeable membrane describes how well. An isotonic solution is the goal of both osmosis and diffusion. If the red cells stay the same size,. Osmolarity and osmolality. Osmolality Isotonic Solution.
From biologydictionary.net
Isotonic vs. Hypotonic vs. Hypertonic Solution Biology Osmolality Isotonic Solution The osmolality of a solution describes how many particles are dissolved in the solution. An isotonic solution is the goal of both osmosis and diffusion. If the red cells stay the same size,. The reflection coefficient of a semipermeable membrane describes how well. This term describes the same concentration of solutes across two areas and is called a. The variance. Osmolality Isotonic Solution.
From www.slideserve.com
PPT Intravenous Therapy IV Infusion Preparations Fluid and Osmolality Isotonic Solution The variance can be significant for concentrated solutions. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. The reflection coefficient of a semipermeable membrane describes how well. If the red cells. Osmolality Isotonic Solution.
From www.researchgate.net
Detachment under changes in solution composition, osmolality, and Osmolality Isotonic Solution Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. The osmolality of a solution describes how many particles are dissolved in the solution. The reflection coefficient of a semipermeable membrane describes how well. The variance can be significant for concentrated solutions. An isotonic solution is the. Osmolality Isotonic Solution.
From www.pinterest.co.uk
Nursing IV Fluid Administration chart. Tonicity, hypertonic, hypotonic Osmolality Isotonic Solution Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. The variance can be significant for concentrated solutions. 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154. Calculated. Osmolality Isotonic Solution.
From ecurrencythailand.com
Can A Solution Be Hyperosmotic And Isotonic? Top Answer Update Osmolality Isotonic Solution Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. The osmolality of a solution describes how many particles are dissolved in the solution. If the red cells stay the same size,. This term describes the same concentration of solutes across two areas and is called a. The reflection coefficient. Osmolality Isotonic Solution.
From pediaa.com
Difference Between Osmolarity and Tonicity Osmolality Isotonic Solution Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. The osmolality of a solution describes how many particles are dissolved in the solution. 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic. Osmolality Isotonic Solution.
From studylib.net
Tonicity, Osmoticity, Osmolarity, & Osmolality Osmolality Isotonic Solution Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. If the red cells stay the same size,. The variance can be significant for concentrated solutions. 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154. An isotonic solution is the goal of both osmosis and. Osmolality Isotonic Solution.
From ibiologia.com
Tonicity Hypotonic, Hyertonic & Isotonic Solutions Osmolality Isotonic Solution An isotonic solution is the goal of both osmosis and diffusion. If the red cells stay the same size,. The osmolality of a solution describes how many particles are dissolved in the solution. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; Osmolality is a colligative property of solutions that depends on the number. Osmolality Isotonic Solution.
From www.studocu.com
Clinical exam uno IV Solutions (2) Isotonic Solutions → total Osmolality Isotonic Solution If the red cells stay the same size,. The osmolality of a solution describes how many particles are dissolved in the solution. The variance can be significant for concentrated solutions. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent; Osmolality is a colligative property of solutions that depends on the number of dissolved particles. Osmolality Isotonic Solution.
From courses.lumenlearning.com
Kinds of Transport Biology for Majors I Osmolality Isotonic Solution The osmolality of a solution describes how many particles are dissolved in the solution. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.[1] the term osmolality expresses concentrations. If the red. Osmolality Isotonic Solution.
From www.chegg.com
Solved Table III Osmolarity Summary Table Isotonic Osmolality Isotonic Solution This term describes the same concentration of solutes across two areas and is called a. 0.9% nacl (normal saline solution, nss) normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154. If the red cells stay the same size,. The variance can be significant for concentrated solutions. Osmolarity. Osmolality Isotonic Solution.
From www.differencebetween.com
Difference Between Tonicity and Osmolarity Compare the Difference Osmolality Isotonic Solution This term describes the same concentration of solutes across two areas and is called a. The variance can be significant for concentrated solutions. The reflection coefficient of a semipermeable membrane describes how well. If the red cells stay the same size,. The osmolality of a solution describes how many particles are dissolved in the solution. Osmolarity and osmolality are units. Osmolality Isotonic Solution.
From www.researchgate.net
The vagus nerve responds to visceral osmolality changes a, Diagram of Osmolality Isotonic Solution If the red cells stay the same size,. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. An isotonic solution is the goal of both osmosis and diffusion. This term describes the same concentration of solutes across two areas and is called a. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308.. Osmolality Isotonic Solution.
From courses.lumenlearning.com
Osmoregulation and Osmotic Balance OpenStax Biology 2e Osmolality Isotonic Solution The osmolality of a solution describes how many particles are dissolved in the solution. If the red cells stay the same size,. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. The reflection coefficient of a semipermeable membrane describes how well. An isotonic solution is the goal of both. Osmolality Isotonic Solution.
From lookfordiagnosis.com
Osmolar Concentration; Ionic Strength; Osmolarity; Osmolality Osmolality Isotonic Solution Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. If the red cells stay the same size,. Calculated osmolarity 0.9% nacl =9/58.4x2x1000= 308. An isotonic solution is the goal of both osmosis and diffusion. This term describes the same concentration of solutes across two areas and is called a.. Osmolality Isotonic Solution.
From www.chegg.com
Solved Can someone please explain this table to me in simple Osmolality Isotonic Solution This term describes the same concentration of solutes across two areas and is called a. Osmolarity and osmolality are units of solute concentration that are often used in reference to biochemistry and body fluids, and. The reflection coefficient of a semipermeable membrane describes how well. The osmolality of a solution describes how many particles are dissolved in the solution. Calculated. Osmolality Isotonic Solution.