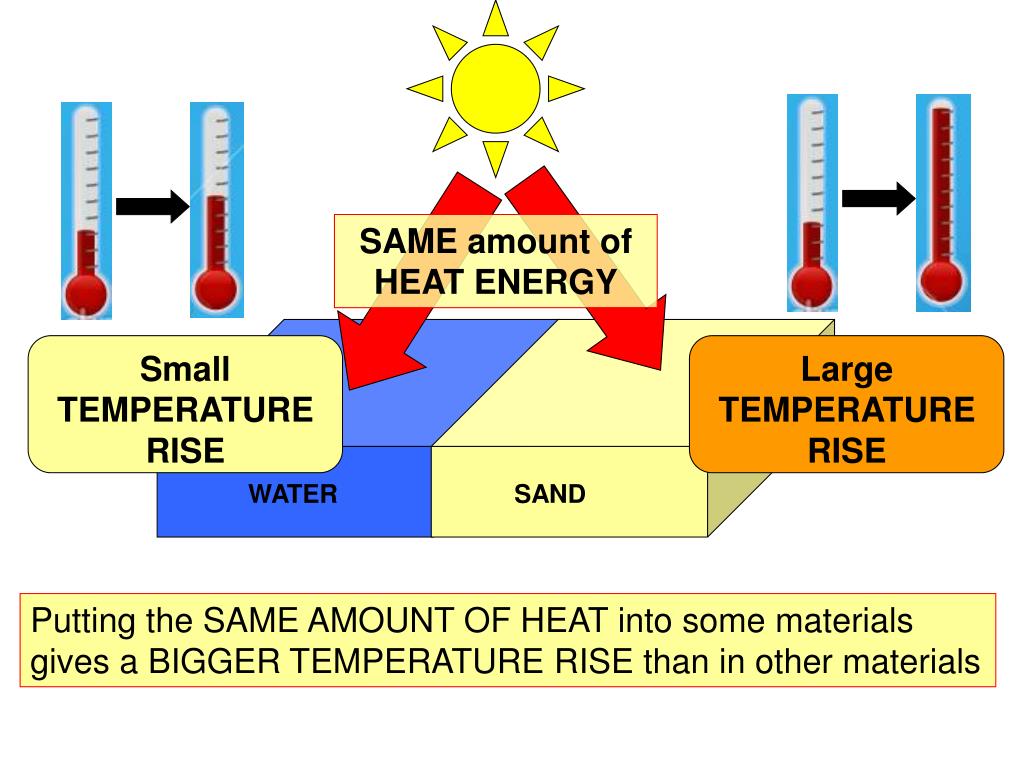
Specific Heat Capacity Nitrogen . The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature. henning and otto, 1936 henning, f.; Calculate the specific heat of an ideal gas for either. When calculating mass and volume flow of a substance in. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. the specific heat of a gas is a measure of the amount of energy necessary to raise the temperature of the gas by a. for polyatomic gases, real or ideal, cv and cp are functions of temperature. l 2 ⋅t −2 ⋅k −1. specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. in the chapter on temperature and heat, we defined the specific heat capacity with the equation q = mcδt, or c = (1 / m)q / δt.
from lessonlibcordwainer.z22.web.core.windows.net
In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit. define heat capacity of an ideal gas for a specific process. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. l 2 ⋅t −2 ⋅k −1. two specific heats are defined for gases, one for constant volume (cv) and one for constant pressure (cp). in the chapter on temperature and heat, we defined the specific heat capacity with the equation q = mcδt, or c = (1 / m)q / δt. Absolute entropy at standard reference pressure. specific heats and gas constants of ideal gases including steam, air, argon and nitrogen are given in the following table. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of.
High Specific Heat Capacity Meaning
Specific Heat Capacity Nitrogen specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. in the chapter on temperature and heat, we defined the specific heat capacity with the equation q = mcδt, or c = (1 / m)q / δt. When calculating mass and volume flow of a substance in. specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature. for polyatomic gases, real or ideal, cv and cp are functions of temperature. Heat capacity is an extensive. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. l 2 ⋅t −2 ⋅k −1. Otto, j., vapor pressure curves and triple points in the temperature region from 14 to 90 k, phys. heat capacity at saturation pressure (crystal 1 in equilibrium with gas) as a function of temperature temperature from 35.61 k to. figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. specific heat of nitrogen is 1.04 j/g k. Absolute entropy at standard reference pressure. define heat capacity of an ideal gas for a specific process. Cp is always greater than cv,. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in.
From www.numerade.com
SOLVED Compute the specific heat capacity at constant volume of Specific Heat Capacity Nitrogen 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. in the chapter on temperature and heat, we defined the specific heat capacity with the equation q = mcδt, or c = (1 / m)q / δt. heat capacity at saturation pressure (crystal 1 in equilibrium with gas). Specific Heat Capacity Nitrogen.
From www.doubtnut.com
Calculate the difference between two specific heats of 1 g of nitrogen Specific Heat Capacity Nitrogen the specific heat of a gas is a measure of the amount of energy necessary to raise the temperature of the gas by a. figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that. Specific Heat Capacity Nitrogen.
From www.youtube.com
Determining the Specific Heat Capacity of Materials GCSE Physics Specific Heat Capacity Nitrogen Heat capacity is an extensive. two specific heats are defined for gases, one for constant volume (cv) and one for constant pressure (cp). Absolute entropy at standard reference pressure. for polyatomic gases, real or ideal, cv and cp are functions of temperature. Otto, j., vapor pressure curves and triple points in the temperature region from 14 to 90. Specific Heat Capacity Nitrogen.
From www.toppr.com
If CP and CV denote the specific heats of nitrogen per unit mass at Specific Heat Capacity Nitrogen Calculate the specific heat of an ideal gas for either. specific heats and gas constants of ideal gases including steam, air, argon and nitrogen are given in the following table. When calculating mass and volume flow of a substance in. Cp is always greater than cv,. two specific heats are defined for gases, one for constant volume (cv). Specific Heat Capacity Nitrogen.
From physicscalculations.com
How to Calculate Specific Heat Capacity Specific Heat Capacity Nitrogen specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature. figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of.. Specific Heat Capacity Nitrogen.
From www.researchgate.net
Figure B.42 (a) Nitrogen density and (b) constant pressure specific Specific Heat Capacity Nitrogen for polyatomic gases, real or ideal, cv and cp are functions of temperature. specific heat of nitrogen is 1.04 j/g k. specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature. specific heats and gas constants of ideal gases including steam, air, argon and. Specific Heat Capacity Nitrogen.
From www.researchgate.net
The temperature dependences of the idealgas specific heat ratio γ0 of Specific Heat Capacity Nitrogen specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. two specific heats are defined for gases, one for constant volume (cv) and one for constant pressure (cp). the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific. Specific Heat Capacity Nitrogen.
From www.toppr.com
An ideal diatomic, gas undergoes a polytropic process described by the Specific Heat Capacity Nitrogen Cp is always greater than cv,. specific heat of nitrogen is 1.04 j/g k. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. Heat capacity is an extensive. specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its. Specific Heat Capacity Nitrogen.
From itrainfitnessgrp.com
Recupera murmurînd Madison heat capacity of nitrogen Doar fao Aerisire Specific Heat Capacity Nitrogen specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature. thermodynamic properties of nitrogen table, specific volume, m3/kg, internal energy, kj/kg, enthalpy, kj/kg, and entropy, kj/kg. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific. Specific Heat Capacity Nitrogen.
From www.youtube.com
Specific heat capacity with hydrogen and nitrogen jee mains 2017 YouTube Specific Heat Capacity Nitrogen two specific heats are defined for gases, one for constant volume (cv) and one for constant pressure (cp). define heat capacity of an ideal gas for a specific process. figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. heat capacity at saturation pressure (crystal 1 in equilibrium with. Specific Heat Capacity Nitrogen.
From dxonmopmx.blob.core.windows.net
Specific Heat Capacity Of Nitrogen Gas At Constant Pressure at Jessica Specific Heat Capacity Nitrogen specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. the specific heat of a gas is a measure of the amount of energy necessary to raise the temperature of. Specific Heat Capacity Nitrogen.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Heat Of Formation Specific Heat Capacity Nitrogen specific heats and gas constants of ideal gases including steam, air, argon and nitrogen are given in the following table. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the. Specific Heat Capacity Nitrogen.
From lessonlibcordwainer.z22.web.core.windows.net
High Specific Heat Capacity Meaning Specific Heat Capacity Nitrogen When calculating mass and volume flow of a substance in. figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. Otto, j., vapor pressure curves and triple points in the temperature region from 14 to 90 k, phys. specific heat is the amount of thermal energy you need to supply to. Specific Heat Capacity Nitrogen.
From dxoqmmcyh.blob.core.windows.net
Heat Capacity Ratio Of Nitrogen Gas at William Parker blog Specific Heat Capacity Nitrogen l 2 ⋅t −2 ⋅k −1. in the chapter on temperature and heat, we defined the specific heat capacity with the equation q = mcδt, or c = (1 / m)q / δt. the specific heat of a gas is a measure of the amount of energy necessary to raise the temperature of the gas by a.. Specific Heat Capacity Nitrogen.
From byjus.com
The SI unit of specific heat capacity of a substance is Specific Heat Capacity Nitrogen Heat capacity is an extensive. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit. Calculate the specific heat of an ideal gas for either. in the chapter on temperature and heat, we defined the specific heat capacity with the equation q = mcδt, or c. Specific Heat Capacity Nitrogen.
From www.slideserve.com
PPT Chapter 17 “Thermochemistry” PowerPoint Presentation, free Specific Heat Capacity Nitrogen specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature. Otto, j., vapor pressure curves and triple points in the temperature region from 14 to 90 k, phys. Cp is always greater than cv,. Heat capacity is an extensive. two specific heats are defined for gases,. Specific Heat Capacity Nitrogen.
From www.chegg.com
Solved TABLE A2 Idealgas specific heats of various common Specific Heat Capacity Nitrogen figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. Heat capacity is an extensive. in the chapter on temperature and heat, we defined the specific heat capacity with the equation q = mcδt, or c = (1 / m)q / δt. 55 rows the table of specific heat capacities. Specific Heat Capacity Nitrogen.
From www.animalia-life.club
Specific Heat Chart Of Common Substances Specific Heat Capacity Nitrogen In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit. specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. the specific heat of a gas is a measure of the amount of energy necessary. Specific Heat Capacity Nitrogen.
From askfilo.com
Calculate the difference between the two specific heats of nitrogen, give.. Specific Heat Capacity Nitrogen for polyatomic gases, real or ideal, cv and cp are functions of temperature. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. in the chapter on temperature and heat, we defined the specific heat capacity with the equation q = mcδt, or c = (1 /. Specific Heat Capacity Nitrogen.
From www1.grc.nasa.gov
Specific Heats cp and cv Glenn Research Center NASA Specific Heat Capacity Nitrogen henning and otto, 1936 henning, f.; In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit. Absolute entropy at standard reference pressure. define heat capacity of an ideal gas for a specific process. Otto, j., vapor pressure curves and triple points in the temperature region. Specific Heat Capacity Nitrogen.
From www.atmo.arizona.edu
Tue., Oct. 6 notes Specific Heat Capacity Nitrogen In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit. thermodynamic properties of nitrogen table, specific volume, m3/kg, internal energy, kj/kg, enthalpy, kj/kg, and entropy, kj/kg. define heat capacity of an ideal gas for a specific process. henning and otto, 1936 henning, f.; . Specific Heat Capacity Nitrogen.
From www.coursehero.com
[Solved] Find the specific heat at constant pressure of nitrogen gas Specific Heat Capacity Nitrogen thermodynamic properties of nitrogen table, specific volume, m3/kg, internal energy, kj/kg, enthalpy, kj/kg, and entropy, kj/kg. the specific heat of a gas is a measure of the amount of energy necessary to raise the temperature of the gas by a. figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units.. Specific Heat Capacity Nitrogen.
From www.researchgate.net
Specific heat in constant volume of the nitrogen versus temperature in Specific Heat Capacity Nitrogen specific heat of nitrogen is 1.04 j/g k. two specific heats are defined for gases, one for constant volume (cv) and one for constant pressure (cp). the specific heat of a gas is a measure of the amount of energy necessary to raise the temperature of the gas by a. specific heats and gas constants of. Specific Heat Capacity Nitrogen.
From physics.stackexchange.com
thermodynamics Liquid nitrogen physical properties Physics Stack Specific Heat Capacity Nitrogen The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. henning and otto, 1936 henning, f.; specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. Cp is always greater than cv,. figures and tables showing thermal diffusivity. Specific Heat Capacity Nitrogen.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Specific Heat Capacity Nitrogen heat capacity at saturation pressure (crystal 1 in equilibrium with gas) as a function of temperature temperature from 35.61 k to. l 2 ⋅t −2 ⋅k −1. specific heats and gas constants of ideal gases including steam, air, argon and nitrogen are given in the following table. specific heat is the amount of heat needed to. Specific Heat Capacity Nitrogen.
From selfstudypoint.in
Specific Heat Capacity Specific Heat Capacity Nitrogen Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. two specific heats are defined for gases, one for constant volume (cv) and one for constant pressure (cp). specific heat of. Specific Heat Capacity Nitrogen.
From mytutorsource.hk
Learn About Specific Heat Capacity in Less Than 10 Minutes! Specific Heat Capacity Nitrogen 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. When calculating mass and volume flow of a substance in. two specific heats are defined for gases, one for constant volume (cv) and one for constant pressure (cp). the specific heat (= specific heat capacity) at constant pressure. Specific Heat Capacity Nitrogen.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Specific Heat Capacity Nitrogen Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. in the. Specific Heat Capacity Nitrogen.
From www.animalia-life.club
Specific Heat Chart Of Common Substances Specific Heat Capacity Nitrogen for polyatomic gases, real or ideal, cv and cp are functions of temperature. specific heats and gas constants of ideal gases including steam, air, argon and nitrogen are given in the following table. heat capacity at saturation pressure (crystal 1 in equilibrium with gas) as a function of temperature temperature from 35.61 k to. Calculate the specific. Specific Heat Capacity Nitrogen.
From exogeegww.blob.core.windows.net
Heating Capacity Of Heat Pump Formula at Lindsey Hazelton blog Specific Heat Capacity Nitrogen When calculating mass and volume flow of a substance in. Absolute entropy at standard reference pressure. the specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats. thermodynamic properties of nitrogen table, specific volume, m3/kg, internal energy, kj/kg, enthalpy, kj/kg, and entropy, kj/kg. for polyatomic gases, real or. Specific Heat Capacity Nitrogen.
From www.engineeringtoolbox.com
Nitrogen Enthalpy, Internal Energy and Entropy vs. Temperature Specific Heat Capacity Nitrogen two specific heats are defined for gases, one for constant volume (cv) and one for constant pressure (cp). for polyatomic gases, real or ideal, cv and cp are functions of temperature. l 2 ⋅t −2 ⋅k −1. figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. heat. Specific Heat Capacity Nitrogen.
From www.studypool.com
SOLUTION Physics notes heat capacity and specific heat capacity Specific Heat Capacity Nitrogen henning and otto, 1936 henning, f.; specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. for polyatomic gases, real or ideal, cv and cp are functions of temperature. thermodynamic properties of nitrogen table, specific volume, m3/kg, internal energy, kj/kg, enthalpy, kj/kg, and entropy, kj/kg. . Specific Heat Capacity Nitrogen.
From www.chegg.com
Solved TABLE A20 Ideal Gas Specific Heats of Some Common Specific Heat Capacity Nitrogen In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit. specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. The specific heat capacity is the amount of heat it takes to change the temperature of. Specific Heat Capacity Nitrogen.
From dxoqmmcyh.blob.core.windows.net
Heat Capacity Ratio Of Nitrogen Gas at William Parker blog Specific Heat Capacity Nitrogen in the chapter on temperature and heat, we defined the specific heat capacity with the equation q = mcδt, or c = (1 / m)q / δt. heat capacity at saturation pressure (crystal 1 in equilibrium with gas) as a function of temperature temperature from 35.61 k to. Otto, j., vapor pressure curves and triple points in the. Specific Heat Capacity Nitrogen.
From oercommons.org
Physical Properties Specific Heat Capacity OER Commons Specific Heat Capacity Nitrogen figures and tables showing thermal diffusivity of nitrogen at varying temperarure and pressure, si and imperial units. specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. l 2 ⋅t −2 ⋅k −1. The specific heat capacity is the amount of heat it takes to change the. Specific Heat Capacity Nitrogen.