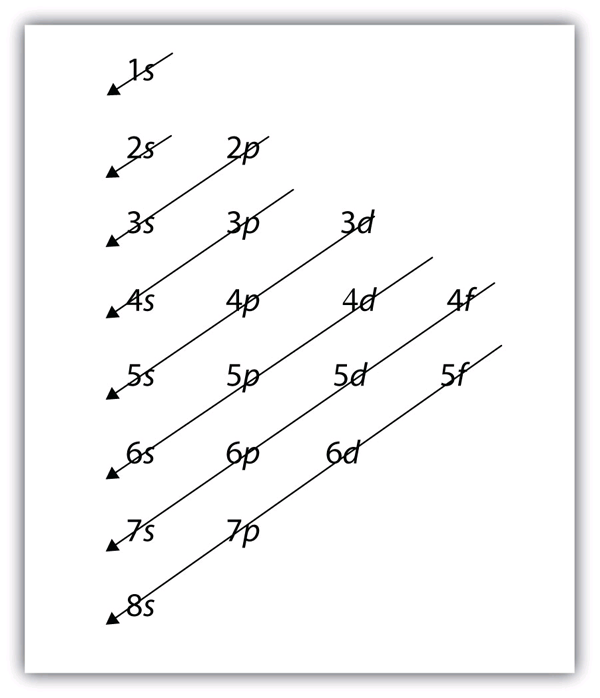
What Is The Meaning Of Electron Shells . Electrons are organized according to their energies into. an electron shell may be thought of as an orbit followed by electrons around an atom nucleus. Because each shell can contain. what are electron shells (energy levels)? Regardless of which electron shell we are talking about,. if you're seeing this message, it means we're having trouble loading external resources on our website. Electrons in atoms are in shells (shown as circles around the nucleus). an electron shell is the space around the nucleus in which an electron can be found. at some point an electron shell will be filled with electrons. the electrons of the outermost energy level determine the energetic stability of the atom and its tendency to form chemical bonds with. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. in simple words, the electron configuration of an atom is the representation of the arrangement of electrons distributed. why do only a certain number of electrons occupy each shell? For many years, it was believed. in very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus.
from courses.lumenlearning.com
electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. why do only a certain number of electrons occupy each shell? Electrons in atoms are in shells (shown as circles around the nucleus). in chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. what are electron shells (energy levels)? electrons orbit the nucleus of an atom at different ranges, called shells. an electron shell may be thought of as an orbit followed by electrons around an atom nucleus. Regardless of which electron shell we are talking about,. at some point an electron shell will be filled with electrons.
Organization of Electrons in Atoms Introductory Chemistry
What Is The Meaning Of Electron Shells Different shells can hold different maximum. in chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. in very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. the bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at. Electrons in atoms occupy energy levels, also called electron shells, outside the. an electron shell is the space around the nucleus in which an electron can be found. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save. Regardless of which electron shell we are talking about,. Electrons are organized according to their energies into. the electrons of the outermost energy level determine the energetic stability of the atom and its tendency to form chemical bonds with. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Each shell has a different energy level, increasing the. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. For many years, it was believed. an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. what are electron shells (energy levels)?
From ar.inspiredpencil.com
Electron Orbital Shells What Is The Meaning Of Electron Shells if you're seeing this message, it means we're having trouble loading external resources on our website. Each shell has a different energy level, increasing the. here's a graphic i use to explain the difference in my general chemistry courses: electrons orbit the nucleus of an atom at different ranges, called shells. an electron shell is a. What Is The Meaning Of Electron Shells.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is The Meaning Of Electron Shells Why are the shells arranged in certain distances from the. electrons orbit the nucleus of an atom at different ranges, called shells. Different shells can hold different maximum. Electrons in atoms occupy energy levels, also called electron shells, outside the. what are electron shells (energy levels)? in chemistry, an electron shell, or energy level, may be imagined. What Is The Meaning Of Electron Shells.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413898 What Is The Meaning Of Electron Shells Electrons are organized according to their energies into. Regardless of which electron shell we are talking about,. why do only a certain number of electrons occupy each shell? Because each shell can contain. an electron shell may be thought of as an orbit followed by electrons around an atom nucleus. in chemistry, an electron shell, or energy. What Is The Meaning Of Electron Shells.
From icdsc.org
How many electrons are in each shell including 3p orbitals What Is The Meaning Of Electron Shells Electrons in atoms are in shells (shown as circles around the nucleus). electron shells or energy levels are the outermost part of an atom surrounding the atomic nucleus. if you're seeing this message, it means we're having trouble loading external resources on our website. here's a graphic i use to explain the difference in my general chemistry. What Is The Meaning Of Electron Shells.
From ar.inspiredpencil.com
Electron Orbital Shells What Is The Meaning Of Electron Shells an electron shell is a collection of orbitals that share the same principal quantum number, representing the energy levels of. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save. Electrons in atoms are in shells (shown as circles around the nucleus). For many years, it was. What Is The Meaning Of Electron Shells.
From www.nagwa.com
Lesson Video Electron Shells Nagwa What Is The Meaning Of Electron Shells what are electron shells (energy levels)? Why are the shells arranged in certain distances from the. in chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the. What Is The Meaning Of Electron Shells.
From mavink.com
Valence Electron Orbital Diagram What Is The Meaning Of Electron Shells in chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. Electrons in atoms occupy energy levels, also called electron shells, outside the. in very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. an electron shell is. What Is The Meaning Of Electron Shells.
From physicsteacher.in
Electron shells for the first 20 elements & shells filling rules What Is The Meaning Of Electron Shells Different shells can hold different maximum. Electrons in atoms occupy energy levels, also called electron shells, outside the. electron shells or energy levels are the outermost part of an atom surrounding the atomic nucleus. here's a graphic i use to explain the difference in my general chemistry courses: why do only a certain number of electrons occupy. What Is The Meaning Of Electron Shells.
From www.britannica.com
Atom Electrons, Nucleus, Bonds Britannica What Is The Meaning Of Electron Shells electron shells or energy levels are the outermost part of an atom surrounding the atomic nucleus. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Because each shell can contain. For many years, it was believed. what are electron shells (energy levels)? in very simple terms an electron shell is the outside. What Is The Meaning Of Electron Shells.
From sciencenotes.org
Periodic Table Showing Shells What Is The Meaning Of Electron Shells revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save. For many years, it was believed. Electrons in atoms are in shells (shown as circles around the nucleus). what are electron shells (energy levels)? electron shells or energy levels are the outermost part of an atom. What Is The Meaning Of Electron Shells.
From oerpub.github.io
This four panel figure shows four different atoms with the electrons in What Is The Meaning Of Electron Shells Because each shell can contain. an electron shell is the space around the nucleus in which an electron can be found. in very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. electron shells or energy levels are the outermost part of an atom surrounding the atomic nucleus. . What Is The Meaning Of Electron Shells.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science What Is The Meaning Of Electron Shells Electrons in atoms are in shells (shown as circles around the nucleus). an electron shell is the space around the nucleus in which an electron can be found. Why are the shells arranged in certain distances from the. Because each shell can contain. here's a graphic i use to explain the difference in my general chemistry courses: . What Is The Meaning Of Electron Shells.
From www.thesciencehive.co.uk
Electron Structure* — the science sauce What Is The Meaning Of Electron Shells revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save. The shells are orbital paths that are. here's a graphic i use to explain the difference in my general chemistry courses: an electron shell, or main energy level, is the part of an atom where electrons. What Is The Meaning Of Electron Shells.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost What Is The Meaning Of Electron Shells Why are the shells arranged in certain distances from the. Because each shell can contain. Different shells can hold different maximum. if you're seeing this message, it means we're having trouble loading external resources on our website. an electron shell is the space around the nucleus in which an electron can be found. revision notes on 1.1.4. What Is The Meaning Of Electron Shells.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Is The Meaning Of Electron Shells the electrons of the outermost energy level determine the energetic stability of the atom and its tendency to form chemical bonds with. in very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. Each shell has a different energy level, increasing the. an electron shell, or main energy level,. What Is The Meaning Of Electron Shells.
From www.animalia-life.club
Electron Shell Diagram What Is The Meaning Of Electron Shells an electron shell may be thought of as an orbit followed by electrons around an atom nucleus. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save. the bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular. What Is The Meaning Of Electron Shells.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Is The Meaning Of Electron Shells revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save. why do only a certain number of electrons occupy each shell? Electrons in atoms occupy energy levels, also called electron shells, outside the. Each shell has a different energy level, increasing the. the electrons of the. What Is The Meaning Of Electron Shells.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is The Meaning Of Electron Shells Electrons are organized according to their energies into. if you're seeing this message, it means we're having trouble loading external resources on our website. For many years, it was believed. in chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. Electrons in atoms occupy energy levels,. What Is The Meaning Of Electron Shells.
From profliway.hubpages.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind What Is The Meaning Of Electron Shells Why are the shells arranged in certain distances from the. what are electron shells (energy levels)? at some point an electron shell will be filled with electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. if you're seeing this message, it means we're having trouble loading external resources on our website. For many. What Is The Meaning Of Electron Shells.
From socratic.org
What is the difference between electron shells and electron orbitals What Is The Meaning Of Electron Shells Electrons in atoms occupy energy levels, also called electron shells, outside the. why do only a certain number of electrons occupy each shell? Each shell has a different energy level, increasing the. The shells are orbital paths that are. the electrons of the outermost energy level determine the energetic stability of the atom and its tendency to form. What Is The Meaning Of Electron Shells.
From courses.lumenlearning.com
Organization of Electrons in Atoms Introductory Chemistry What Is The Meaning Of Electron Shells in chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. an electron shell may be thought of as an orbit followed by electrons around an atom nucleus. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry. What Is The Meaning Of Electron Shells.
From lessondbchandelles.z21.web.core.windows.net
Bohr's Atomic Model Notes What Is The Meaning Of Electron Shells Each shell has a different energy level, increasing the. revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save. electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. here's a graphic i use to explain the difference in my general chemistry courses:. What Is The Meaning Of Electron Shells.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science What Is The Meaning Of Electron Shells For many years, it was believed. here's a graphic i use to explain the difference in my general chemistry courses: Why are the shells arranged in certain distances from the. in very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. Electrons are organized according to their energies into. . What Is The Meaning Of Electron Shells.
From www.slideserve.com
PPT BIOLOGY EOC REVIEW PowerPoint Presentation, free download ID What Is The Meaning Of Electron Shells For many years, it was believed. in simple words, the electron configuration of an atom is the representation of the arrangement of electrons distributed. Different shells can hold different maximum. Regardless of which electron shell we are talking about,. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the. What Is The Meaning Of Electron Shells.
From commons.wikimedia.org
FileElectron shell 079 gold.png Wikimedia Commons What Is The Meaning Of Electron Shells Different shells can hold different maximum. at some point an electron shell will be filled with electrons. Regardless of which electron shell we are talking about,. electron shells or energy levels are the outermost part of an atom surrounding the atomic nucleus. For many years, it was believed. the bohr model shows the atom as a central. What Is The Meaning Of Electron Shells.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Is The Meaning Of Electron Shells why do only a certain number of electrons occupy each shell? Regardless of which electron shell we are talking about,. Each shell has a different energy level, increasing the. an electron shell may be thought of as an orbit followed by electrons around an atom nucleus. in chemistry, an electron shell, or energy level, may be imagined. What Is The Meaning Of Electron Shells.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png What Is The Meaning Of Electron Shells in very simple terms an electron shell is the outside part of an atom that surrounds the atomic nucleus. why do only a certain number of electrons occupy each shell? the bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at. an electron shell is the. What Is The Meaning Of Electron Shells.
From biologydictionary.net
Covalent Bond Biology Dictionary What Is The Meaning Of Electron Shells an electron shell is the space around the nucleus in which an electron can be found. Electrons in atoms occupy energy levels, also called electron shells, outside the. at some point an electron shell will be filled with electrons. if you're seeing this message, it means we're having trouble loading external resources on our website. electron. What Is The Meaning Of Electron Shells.
From www.animalia-life.club
Electron Shell Diagram What Is The Meaning Of Electron Shells electrons orbit the nucleus of an atom at different ranges, called shells. Because each shell can contain. what are electron shells (energy levels)? an electron shell is the space around the nucleus in which an electron can be found. at some point an electron shell will be filled with electrons. if you're seeing this message,. What Is The Meaning Of Electron Shells.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free What Is The Meaning Of Electron Shells Different shells can hold different maximum. if you're seeing this message, it means we're having trouble loading external resources on our website. Why are the shells arranged in certain distances from the. The shells are orbital paths that are. Regardless of which electron shell we are talking about,. an electron shell is the space around the nucleus in. What Is The Meaning Of Electron Shells.
From commons.wikimedia.org
FileElectron shell 019 potassium.png Wikimedia Commons What Is The Meaning Of Electron Shells revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save. Because each shell can contain. in chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. Electrons are organized according to their energies into. if. What Is The Meaning Of Electron Shells.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is The Meaning Of Electron Shells Electrons in atoms occupy energy levels, also called electron shells, outside the. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. the bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at. an electron shell. What Is The Meaning Of Electron Shells.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Meaning Of Electron Shells here's a graphic i use to explain the difference in my general chemistry courses: Electrons in atoms occupy energy levels, also called electron shells, outside the. Because each shell can contain. an electron shell, or main energy level, is the part of an atom where electrons are found orbiting the atom's nucleus. Regardless of which electron shell we. What Is The Meaning Of Electron Shells.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is The Meaning Of Electron Shells Electrons in atoms are in shells (shown as circles around the nucleus). why do only a certain number of electrons occupy each shell? revision notes on 1.1.4 shells and orbitals for the aqa a level chemistry syllabus, written by the chemistry experts at save. electron shells or energy levels are the outermost part of an atom surrounding. What Is The Meaning Of Electron Shells.
From www.nagwa.com
Question Video Finding the Number of Additional Electrons an Electron What Is The Meaning Of Electron Shells For many years, it was believed. Each shell has a different energy level, increasing the. Electrons are organized according to their energies into. in chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The shells are orbital paths that are. an electron shell is the space. What Is The Meaning Of Electron Shells.