Indicator Of Electric Potential . Potentiometric titration is a laboratory method to determine the concentration of a given analyte. 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: Define electric potential and electric potential energy. In this method, there is no use of a chemical indicator. Metallic electrodes, which are the subject of this section, and ion. Describe the relationship between electric potential difference and electric field. A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. It is used in the characterization of acids. Two classes of indicator electrodes are used to make potentiometric measurements: Instead, the electric potential across the substance is measured.
from www.onenewspage.com
A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. In this method, there is no use of a chemical indicator. Define electric potential and electric potential energy. Two classes of indicator electrodes are used to make potentiometric measurements: Metallic electrodes, which are the subject of this section, and ion. It is used in the characterization of acids. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: Instead, the electric potential across the substance is measured. 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /.
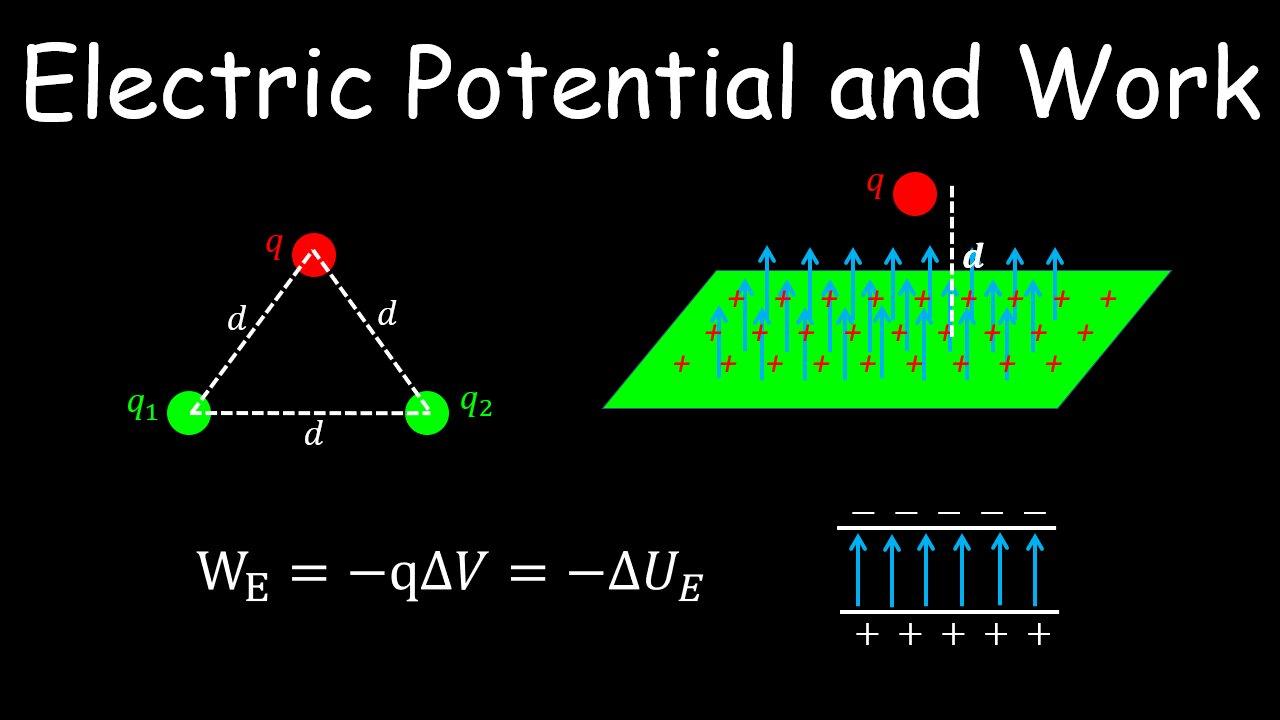
Electric Potential and Work, Electrostatics One News Page VIDEO
Indicator Of Electric Potential A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. Metallic electrodes, which are the subject of this section, and ion. In this method, there is no use of a chemical indicator. Two classes of indicator electrodes are used to make potentiometric measurements: Instead, the electric potential across the substance is measured. Define electric potential and electric potential energy. Describe the relationship between electric potential difference and electric field. It is used in the characterization of acids. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the.
From quizlet.com
What is the electric potential at the point indicated with t Quizlet Indicator Of Electric Potential The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: Define electric potential and electric potential energy. Metallic electrodes, which are the subject of this section, and ion. Describe the relationship between electric potential. Indicator Of Electric Potential.
From www.researchgate.net
(a) Stages of electrical potential dynamics period 'A' is a normal Indicator Of Electric Potential It is used in the characterization of acids. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. The potential of. Indicator Of Electric Potential.
From www.researchgate.net
Potential measurement parameters and fault indicators in electrical Indicator Of Electric Potential It is used in the characterization of acids. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: In this method, there is no use of a chemical indicator. Two classes of indicator electrodes are used to make potentiometric measurements: Define electric potential and electric potential energy. Metallic electrodes, which are the. Indicator Of Electric Potential.
From www.animalia-life.club
Electric Potential Energy Diagram Indicator Of Electric Potential Metallic electrodes, which are the subject of this section, and ion. In this method, there is no use of a chemical indicator. A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. 9 rows electric. Indicator Of Electric Potential.
From www.slideserve.com
PPT Chapter 25 Electric potential 251 Potential Difference and Indicator Of Electric Potential A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. It is used in the characterization of. Indicator Of Electric Potential.
From www.britannica.com
Electric potential Definition, Facts, & Units Britannica Indicator Of Electric Potential 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. Metallic electrodes, which are the subject of this section, and ion. Describe the relationship between electric potential difference and electric field. Two classes of indicator electrodes are used to make potentiometric measurements: The potential of an indicator. Indicator Of Electric Potential.
From simply.science
Electrochemistry Indicator Of Electric Potential A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Two classes of indicator electrodes are used to make potentiometric measurements:. Indicator Of Electric Potential.
From memorang.com
Electric potential explainer Flashcards Memorang Indicator Of Electric Potential Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. In this method, there is no use of a chemical indicator. 9 rows electric potential (also called the electric field potential, potential drop, the. Indicator Of Electric Potential.
From www.teachoo.com
Electric Potential Difference Definition, Formula, Unit Teachoo Indicator Of Electric Potential Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Two classes of indicator electrodes are used to make potentiometric measurements: The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. Instead, the electric potential across the substance is measured. A comparison between the potential that an indicator. Indicator Of Electric Potential.
From studylib.net
ELECTRIC POTENTIALENERGY (U) and the ELECTRIC Indicator Of Electric Potential Describe the relationship between electric potential difference and electric field. Metallic electrodes, which are the subject of this section, and ion. A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. 9 rows electric potential. Indicator Of Electric Potential.
From www.researchgate.net
Electric potential distribution and current density vector field 3D Indicator Of Electric Potential It is used in the characterization of acids. Define electric potential and electric potential energy. 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. Two classes of indicator electrodes are used to make potentiometric measurements: The potential of an indicator electrode is related to the concentration. Indicator Of Electric Potential.
From www.animalia-life.club
Electric Potential Energy Diagram Indicator Of Electric Potential Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Two classes of indicator electrodes are used to make potentiometric measurements: Metallic electrodes, which are the subject of this section, and ion. 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. Define. Indicator Of Electric Potential.
From www.doubtnut.com
What is the electric potential at a point P, distance r from the midp Indicator Of Electric Potential In this method, there is no use of a chemical indicator. Describe the relationship between electric potential difference and electric field. Metallic electrodes, which are the subject of this section, and ion. Two classes of indicator electrodes are used to make potentiometric measurements: Define electric potential and electric potential energy. It is used in the characterization of acids. Direct potentiometric. Indicator Of Electric Potential.
From eduinput.com
Electric Potential Electric Field as Potential Gradient Indicator Of Electric Potential Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: Metallic electrodes, which are the subject of this section, and ion. In this method, there is no use of a chemical indicator. 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount. Indicator Of Electric Potential.
From courses.lumenlearning.com
Electric Potential Energy Potential Difference Physics Indicator Of Electric Potential In this method, there is no use of a chemical indicator. Metallic electrodes, which are the subject of this section, and ion. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: It is used in the characterization of acids. Describe the relationship between electric potential difference and electric field. Define electric. Indicator Of Electric Potential.
From www.researchgate.net
Electrical potential profiles from 20 June 2004. Download Scientific Indicator Of Electric Potential Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Describe the relationship between electric. Indicator Of Electric Potential.
From www.researchgate.net
Electrical potential at 4.5 kV/25 kHz (with flow). Download Indicator Of Electric Potential Metallic electrodes, which are the subject of this section, and ion. Two classes of indicator electrodes are used to make potentiometric measurements: 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. Describe the relationship between electric potential difference and electric field. It is used in the. Indicator Of Electric Potential.
From www.industrialservomotor.com
KC241 1282721 Electrical Potential Indicator Dual Scale Illumination Indicator Of Electric Potential In this method, there is no use of a chemical indicator. Describe the relationship between electric potential difference and electric field. The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. It is used in the characterization of acids. Direct potentiometric measurements offer a quick and practical way to assess the activity. Indicator Of Electric Potential.
From www.test-meter.co.uk
HV Detectors & Potential Indicators for 3kV 400kV electrical systems Indicator Of Electric Potential Instead, the electric potential across the substance is measured. The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. Describe the relationship between electric potential difference and electric field. Two classes of indicator electrodes are used to make potentiometric measurements: Potentiometric titration is a laboratory method to determine the concentration of a. Indicator Of Electric Potential.
From app.jove.com
Electric Potential and Potential Difference Concept Physics JoVe Indicator Of Electric Potential Metallic electrodes, which are the subject of this section, and ion. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: Define electric potential and electric potential energy. Instead, the electric potential across the substance is measured. 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential). Indicator Of Electric Potential.
From studylib.net
Ch 17 Electric Potential Indicator Of Electric Potential Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: Describe the relationship between electric potential difference and electric field. Instead, the electric potential across the substance is measured. Two classes of indicator electrodes are used to make potentiometric measurements: Potentiometric titration is a laboratory method to determine the concentration of a. Indicator Of Electric Potential.
From eduinput.com
Electric Potential Electric Field as Potential Gradient Indicator Of Electric Potential Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. It is used in the characterization of acids. A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and. Indicator Of Electric Potential.
From www.slideserve.com
PPT Electric Potential PowerPoint Presentation, free download ID361030 Indicator Of Electric Potential Instead, the electric potential across the substance is measured. Metallic electrodes, which are the subject of this section, and ion. 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and. Indicator Of Electric Potential.
From readaxis.com
Electric Potential Class 10 Detailed Explanation ReadAxis Indicator Of Electric Potential Metallic electrodes, which are the subject of this section, and ion. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. Two classes of indicator electrodes are used to make potentiometric measurements: Define electric potential and electric potential. Indicator Of Electric Potential.
From grupovazsir.com
Electrical Potential Indicator 04000 RPM Grupo Vazsir Indicator Of Electric Potential Two classes of indicator electrodes are used to make potentiometric measurements: Metallic electrodes, which are the subject of this section, and ion. A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte. Instead, the electric. Indicator Of Electric Potential.
From www.studocu.com
Electric potential good for practice Electric Potential (V) Work Indicator Of Electric Potential Instead, the electric potential across the substance is measured. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. In this method, there is no use of a chemical indicator. Define electric potential and electric potential energy. It is used in the characterization of acids. A comparison between the potential that an indicator electrode develops in. Indicator Of Electric Potential.
From www.electricity-magnetism.org
Electric Potential Energy Definition, Formula & Calculation Indicator Of Electric Potential Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The potential of an indicator electrode is related to the concentration of the substance being measured, whereas the. Two classes of indicator electrodes are used to make potentiometric measurements: Metallic electrodes, which are the subject of this section, and ion. It is used in the characterization. Indicator Of Electric Potential.
From www.esaral.com
Mind Maps for Electrostatics Electric Potential Revision Class XII Indicator Of Electric Potential Describe the relationship between electric potential difference and electric field. 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. A comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one. Indicator Of Electric Potential.
From www.slideserve.com
PPT Chapter 25 Electric Potential PowerPoint Presentation, free Indicator Of Electric Potential Instead, the electric potential across the substance is measured. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: Describe the relationship between electric potential difference and electric field. Metallic electrodes, which are the subject of this section,. Indicator Of Electric Potential.
From morioh.com
Electric Potential Indicator Of Electric Potential Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: Potentiometric titration is a laboratory method to determine the concentration of a given analyte. In this method, there is no use of a chemical indicator. The potential of an indicator electrode is related to the concentration of the substance being measured, whereas. Indicator Of Electric Potential.
From www.onenewspage.com
Electric Potential and Work, Electrostatics One News Page VIDEO Indicator Of Electric Potential Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: It is used in the characterization of acids. 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. The potential of an indicator electrode is related to the concentration. Indicator Of Electric Potential.
From studylib.net
Electric Potential Difference Indicator Of Electric Potential Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Describe the relationship between electric potential difference and electric field. It is used in the characterization of acids. Two classes of indicator electrodes are used to make potentiometric measurements: 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined. Indicator Of Electric Potential.
From readaxis.com
Electric Potential Class 10 Detailed Explanation ReadAxis Indicator Of Electric Potential 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. It is used in the characterization of acids. Describe the relationship between electric potential difference and electric field. Define electric potential and electric potential energy. Direct potentiometric measurements offer a quick and practical way to assess the. Indicator Of Electric Potential.
From quizlet.com
What is the electric potential at the point indicated with t Quizlet Indicator Of Electric Potential Two classes of indicator electrodes are used to make potentiometric measurements: Metallic electrodes, which are the subject of this section, and ion. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: Define electric potential and electric potential. Indicator Of Electric Potential.
From www.slideserve.com
PPT Chapter 25 Electric potential 251 Potential Difference and Indicator Of Electric Potential 9 rows electric potential (also called the electric field potential, potential drop, the electrostatic potential) is defined as the amount of work /. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: In this method, there is no use of a chemical indicator. Describe the relationship between electric potential difference and. Indicator Of Electric Potential.