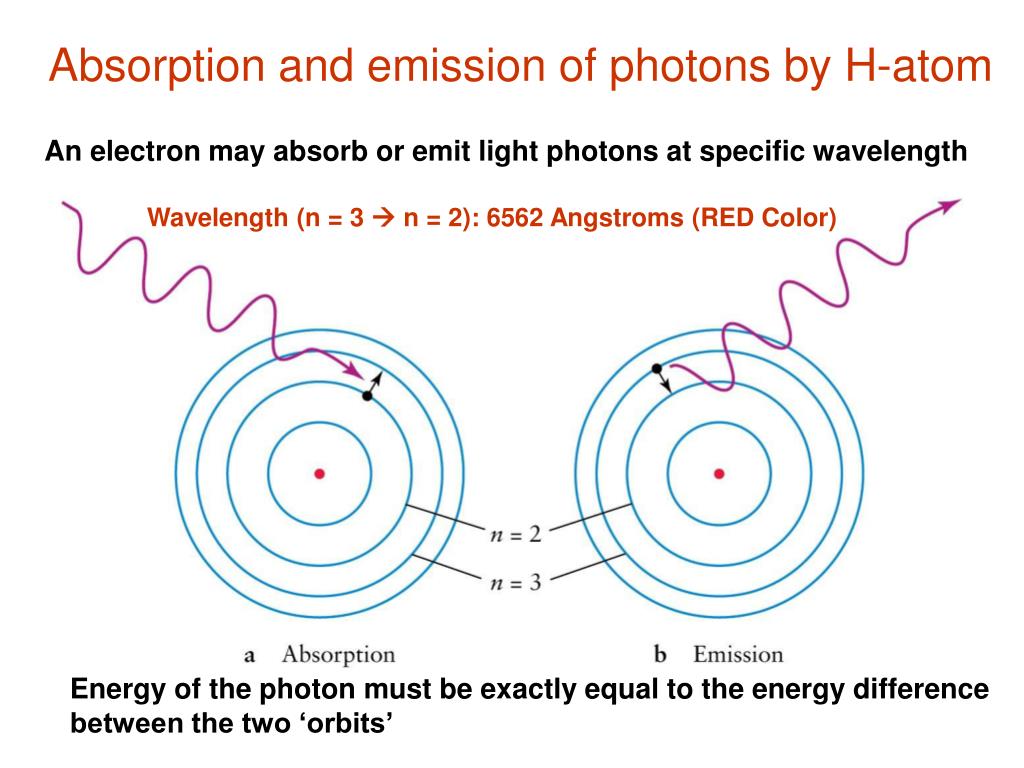
Photons Can Be Emitted But Cannot Be Absorbed . Downward transition from higher energy levels to lower energy levels results in the emission of photons. But the quanta from the photons will be stored partially on the. Emitted and absorbed photons don't change the particles charge. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. The atom can be raised to an excited state by the absorption of a photon. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. When this happens, their light is transformed into heat, warming the matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. For example, if a red photon of frequency \(f\). Photons can be absorbed by matter.
from joiamhcyi.blob.core.windows.net
For example, if a red photon of frequency \(f\). But the quanta from the photons will be stored partially on the. When this happens, their light is transformed into heat, warming the matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed by matter. Emitted and absorbed photons don't change the particles charge. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. Downward transition from higher energy levels to lower energy levels results in the emission of photons. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so.
Can Photons Hit Each Other at Donna Gore blog
Photons Can Be Emitted But Cannot Be Absorbed When this happens, their light is transformed into heat, warming the matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. When this happens, their light is transformed into heat, warming the matter. Downward transition from higher energy levels to lower energy levels results in the emission of photons. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. But the quanta from the photons will be stored partially on the. Emitted and absorbed photons don't change the particles charge. Photons can be absorbed by matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. For example, if a red photon of frequency \(f\). Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. The atom can be raised to an excited state by the absorption of a photon.
From energywavetheory.com
Photon Creation and Absorption EWT Photons Can Be Emitted But Cannot Be Absorbed When this happens, their light is transformed into heat, warming the matter. Downward transition from higher energy levels to lower energy levels results in the emission of photons. Photons can be absorbed by matter. But the quanta from the photons will be stored partially on the. For example, if a red photon of frequency \(f\). The atom can be raised. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideserve.com
PPT Light and Color PowerPoint Presentation, free download ID9081959 Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed by matter. The atom can be raised to an excited state by the absorption of a photon. Emitted and absorbed photons don't change the particles charge. Downward transition from higher energy levels to lower energy levels results in the emission of photons. But the quanta from the photons will be stored partially on the. Photons can. Photons Can Be Emitted But Cannot Be Absorbed.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube Photons Can Be Emitted But Cannot Be Absorbed When this happens, their light is transformed into heat, warming the matter. But the quanta from the photons will be stored partially on the. For example, if a red photon of frequency \(f\). Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Downward transition from higher energy. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideserve.com
PPT Photoelectric effect, photons PowerPoint Presentation, free Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Emitted and absorbed photons don't change the particles charge. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed or emitted only. Photons Can Be Emitted But Cannot Be Absorbed.
From slidetodoc.com
Chapter 18 BoseEinstein Gases 18 1 Blackbody Radiation Photons Can Be Emitted But Cannot Be Absorbed Downward transition from higher energy levels to lower energy levels results in the emission of photons. When this happens, their light is transformed into heat, warming the matter. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized. Photons Can Be Emitted But Cannot Be Absorbed.
From joiamhcyi.blob.core.windows.net
Can Photons Hit Each Other at Donna Gore blog Photons Can Be Emitted But Cannot Be Absorbed The atom can be raised to an excited state by the absorption of a photon. For example, if a red photon of frequency \(f\). Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Single atoms can absorb energy from a photon and store it in an electron—but. Photons Can Be Emitted But Cannot Be Absorbed.
From www.neurotar.com
TwoPhoton Microscopy Neurotar Photons Can Be Emitted But Cannot Be Absorbed The atom can be raised to an excited state by the absorption of a photon. But the quanta from the photons will be stored partially on the. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Single atoms can absorb energy from a photon and store it. Photons Can Be Emitted But Cannot Be Absorbed.
From loepetamz.blob.core.windows.net
How Is A Photon Absorbed at Phillip Martinson blog Photons Can Be Emitted But Cannot Be Absorbed For example, if a red photon of frequency \(f\). The atom can be raised to an excited state by the absorption of a photon. But the quanta from the photons will be stored partially on the. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can. Photons Can Be Emitted But Cannot Be Absorbed.
From joiamhcyi.blob.core.windows.net
Can Photons Hit Each Other at Donna Gore blog Photons Can Be Emitted But Cannot Be Absorbed The atom can be raised to an excited state by the absorption of a photon. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. But the quanta from the photons will be stored partially on the. Emitted and absorbed photons don't change the particles charge. When this. Photons Can Be Emitted But Cannot Be Absorbed.
From slideplayer.com
Properties of Radiation Chapter 5 ppt download Photons Can Be Emitted But Cannot Be Absorbed Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Downward transition from higher energy. Photons Can Be Emitted But Cannot Be Absorbed.
From slideplayer.com
Homework 3 will be posted on Wednesday ppt download Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. When this happens, their light is transformed into heat, warming the matter. For example, if a red photon of frequency \(f\). But the quanta from the photons will be stored partially on the. Single atoms can absorb energy. Photons Can Be Emitted But Cannot Be Absorbed.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. When this happens, their light is transformed into heat, warming the matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideshare.net
Interaction of photons with matter Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed by matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Downward transition from higher energy levels to lower energy levels. Photons Can Be Emitted But Cannot Be Absorbed.
From slideplayer.com
Radiation Balance. ppt download Photons Can Be Emitted But Cannot Be Absorbed The atom can be raised to an excited state by the absorption of a photon. Downward transition from higher energy levels to lower energy levels results in the emission of photons. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideserve.com
PPT Photoelectric effect, photons PowerPoint Presentation, free Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed by matter. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. Downward transition from higher energy levels to lower energy levels results in the emission of photons. Emitted and absorbed photons don't. Photons Can Be Emitted But Cannot Be Absorbed.
From schoolbag.info
Rutherford, Planck, and Bohr Atomic Structure Review Training Photons Can Be Emitted But Cannot Be Absorbed Downward transition from higher energy levels to lower energy levels results in the emission of photons. The atom can be raised to an excited state by the absorption of a photon. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Emitted and absorbed photons don't change the. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideserve.com
PPT Light and Color PowerPoint Presentation, free download ID6899593 Photons Can Be Emitted But Cannot Be Absorbed Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. Downward transition from higher energy levels to lower energy levels results in the emission of photons. Photons can be absorbed by matter. Photons can be absorbed or. Photons Can Be Emitted But Cannot Be Absorbed.
From mavink.com
Photon Diagram Photons Can Be Emitted But Cannot Be Absorbed Downward transition from higher energy levels to lower energy levels results in the emission of photons. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed by matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideserve.com
PPT Chapter 38 Photons and Matter Waves PowerPoint Presentation, free Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Downward transition from higher energy levels to lower energy levels results in the emission of photons. The atom can be raised to an excited state by the absorption of a photon. Single atoms can absorb energy from a. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideserve.com
PPT Chapter 5 Light PowerPoint Presentation, free download ID2768132 Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. But the quanta from the photons will be stored partially on the. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to. Photons Can Be Emitted But Cannot Be Absorbed.
From slideplayer.com
Electrons in Atoms Chapter 5 ppt download Photons Can Be Emitted But Cannot Be Absorbed Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. When this happens, their light is transformed into heat, warming the matter. Downward transition from higher energy levels to lower energy levels results in the emission of. Photons Can Be Emitted But Cannot Be Absorbed.
From courses.lumenlearning.com
Photon Momentum Physics Photons Can Be Emitted But Cannot Be Absorbed The atom can be raised to an excited state by the absorption of a photon. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. Photons can be absorbed or emitted only by atoms and molecules that. Photons Can Be Emitted But Cannot Be Absorbed.
From www.sciencenews.org
Colliding photons made matter. But are the photons ‘real’? Science News Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. When this happens, their light is transformed into heat, warming the matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed. Photons Can Be Emitted But Cannot Be Absorbed.
From www.youtube.com
ABC Zoom Electrons and photons absorption and transmission of light Photons Can Be Emitted But Cannot Be Absorbed The atom can be raised to an excited state by the absorption of a photon. But the quanta from the photons will be stored partially on the. Downward transition from higher energy levels to lower energy levels results in the emission of photons. Photons can be absorbed by matter. When this happens, their light is transformed into heat, warming the. Photons Can Be Emitted But Cannot Be Absorbed.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Photons Can Be Emitted But Cannot Be Absorbed But the quanta from the photons will be stored partially on the. For example, if a red photon of frequency \(f\). Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Downward transition from higher energy levels to lower energy levels results in the emission of photons. The. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideshare.net
Photon and energy levels Photons Can Be Emitted But Cannot Be Absorbed The atom can be raised to an excited state by the absorption of a photon. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. For example, if a red photon of frequency \(f\). Downward transition from higher energy levels to lower energy levels results in the emission. Photons Can Be Emitted But Cannot Be Absorbed.
From chem.libretexts.org
6.2 Quantized Energy and Photons Chemistry LibreTexts Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed by matter. But the quanta from the photons will be stored partially on the. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideserve.com
PPT Chapter 42 PowerPoint Presentation, free download ID5580472 Photons Can Be Emitted But Cannot Be Absorbed But the quanta from the photons will be stored partially on the. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Emitted and absorbed photons don't change the particles charge. Downward transition from higher energy levels to lower energy levels results in the emission of photons. The. Photons Can Be Emitted But Cannot Be Absorbed.
From energywavetheory.com
What is Quantum? EWT Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Emitted and absorbed photons don't change the particles charge. When this happens, their light is transformed. Photons Can Be Emitted But Cannot Be Absorbed.
From pages.uoregon.edu
Astronomy 122 Spectroscopy Photons Can Be Emitted But Cannot Be Absorbed Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. For example, if a red photon of frequency \(f\). But the quanta from the photons will be stored partially on the. Photons can be absorbed or emitted. Photons Can Be Emitted But Cannot Be Absorbed.
From www.slideserve.com
PPT Photoelectric effect, photons PowerPoint Presentation, free Photons Can Be Emitted But Cannot Be Absorbed The atom can be raised to an excited state by the absorption of a photon. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. Photons can be absorbed or emitted only by atoms and molecules that. Photons Can Be Emitted But Cannot Be Absorbed.
From rwu.pressbooks.pub
Chapter 12. Photosynthesis Introduction to Molecular and Cell Biology Photons Can Be Emitted But Cannot Be Absorbed Photons can be absorbed by matter. Single atoms can absorb energy from a photon and store it in an electron—but only if the photon carries just the right amount of energy to match the difference between two quantized energy. Downward transition from higher energy levels to lower energy levels results in the emission of photons. Photons can be absorbed or. Photons Can Be Emitted But Cannot Be Absorbed.
From www.britannica.com
Compton effect Definition, Formula, & Facts Britannica Photons Can Be Emitted But Cannot Be Absorbed Downward transition from higher energy levels to lower energy levels results in the emission of photons. But the quanta from the photons will be stored partially on the. When this happens, their light is transformed into heat, warming the matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to. Photons Can Be Emitted But Cannot Be Absorbed.
From milagroskruwluna.blogspot.com
Describe How Plants Absorb Photons of Light Energy. MilagroskruwLuna Photons Can Be Emitted But Cannot Be Absorbed For example, if a red photon of frequency \(f\). When this happens, their light is transformed into heat, warming the matter. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. The atom can be raised to an excited state by the absorption of a photon. Single atoms. Photons Can Be Emitted But Cannot Be Absorbed.
From www.britannica.com
Light Emission, Absorption, Processes Britannica Photons Can Be Emitted But Cannot Be Absorbed For example, if a red photon of frequency \(f\). But the quanta from the photons will be stored partially on the. Photons can be absorbed or emitted only by atoms and molecules that have precisely the correct quantized energy step to do so. Downward transition from higher energy levels to lower energy levels results in the emission of photons. Photons. Photons Can Be Emitted But Cannot Be Absorbed.