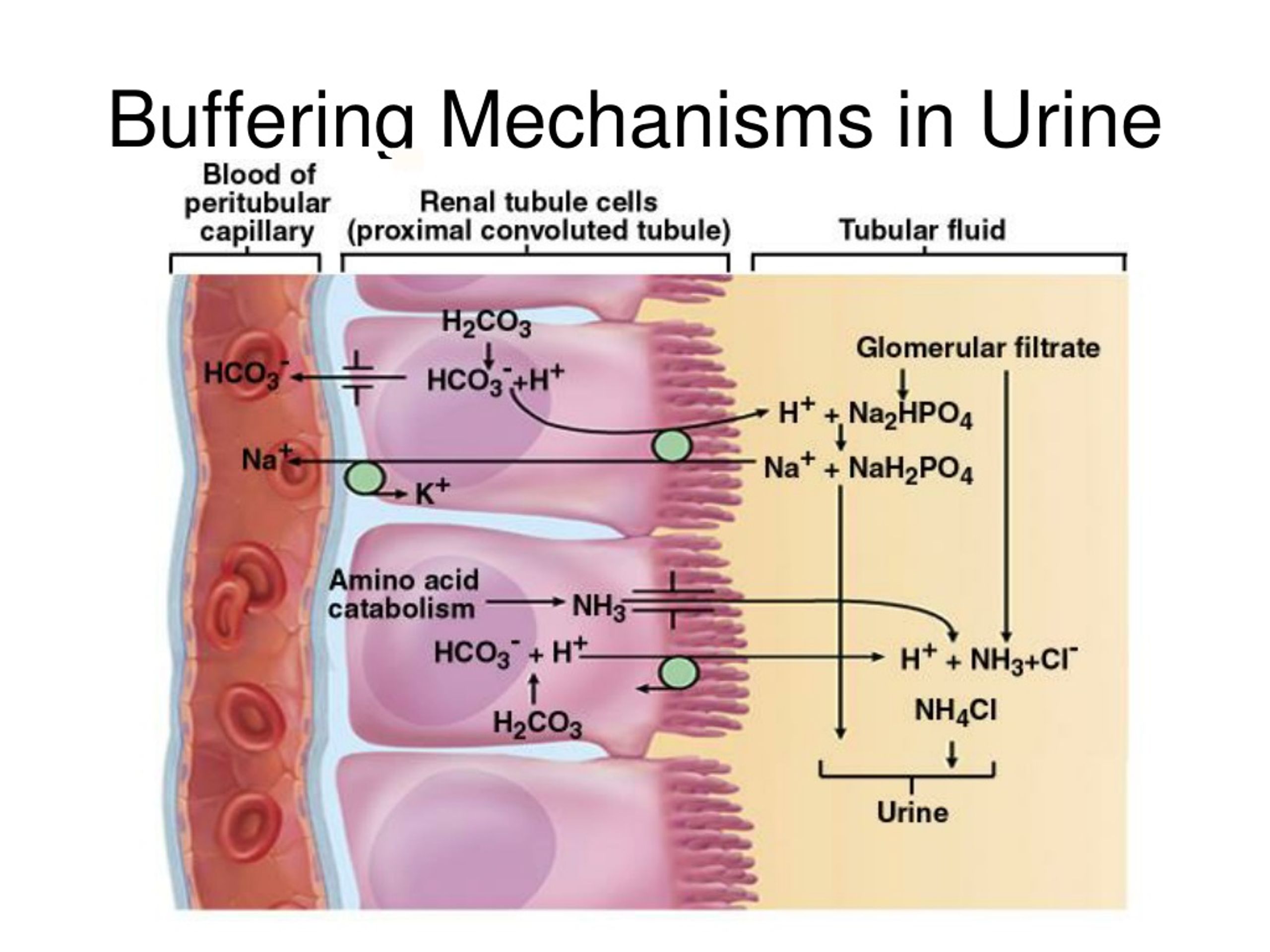
Phosphate Buffer System In Kidney . In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. Disturbances of these mechanisms can have serious clinical consequences. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. All of these contain bases that. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin.
from www.slideserve.com
All of these contain bases that. Disturbances of these mechanisms can have serious clinical consequences. The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss.
PPT Compounds PowerPoint Presentation, free download ID
Phosphate Buffer System In Kidney Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. All of these contain bases that. The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. Disturbances of these mechanisms can have serious clinical consequences.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Phosphate Buffer System In Kidney Disturbances of these mechanisms can have serious clinical consequences. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. In the nephron, the proximal convoluted tubule cells are able to reabsorb the. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT (Renal Physiology 10) AcidBase Balance 2 Buffers System Phosphate Buffer System In Kidney Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion. Phosphate Buffer System In Kidney.
From journals.physiology.org
The kidney and acidbase regulation Advances in Physiology Education Phosphate Buffer System In Kidney The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Bicarbonate is the predominant extracellular buffer against the fixed. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Chapter 24 Water, Electrolyte and AcidBase Balance PowerPoint Phosphate Buffer System In Kidney Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Disturbances of these mechanisms can have serious. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Phosphate Buffer System In Kidney In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Bicarbonate is. Phosphate Buffer System In Kidney.
From doctorlib.info
Acidbase Balance Physiology An Illustrated Review Phosphate Buffer System In Kidney Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain bases that. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions,. Phosphate Buffer System In Kidney.
From labpedia.net
Acidbase Balance Part 1 A Introduction to the AcidBase Balance Phosphate Buffer System In Kidney Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. Disturbances of these mechanisms can have serious clinical consequences. Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to. Phosphate Buffer System In Kidney.
From doctorlib.info
Phosphate Transport of Urea, Glucose, Phosphate, Calcium, Magnesium Phosphate Buffer System In Kidney To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal. Phosphate Buffer System In Kidney.
From slideplayer.com
AcidBase Regulation1 Lecture 8 (9/4/2015) ppt download Phosphate Buffer System In Kidney Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. All of these contain bases that. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. The kidneys are slower to. Phosphate Buffer System In Kidney.
From slideplayer.com
Chapter 24 *APR Lecture PowerPoint ppt download Phosphate Buffer System In Kidney The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Disturbances of these mechanisms can have serious clinical consequences. The principal. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Phosphate Buffer System In Kidney To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The kidneys are slower to compensate than. Phosphate Buffer System In Kidney.
From www.anaesthesiajournal.co.uk
Renal physiology acidbase balance Anaesthesia & Intensive Care Medicine Phosphate Buffer System In Kidney In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. Disturbances of these mechanisms can have serious clinical consequences. The kidneys are slower to compensate than the. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Chapter 4 Acidbase balance and acidbase disorders PowerPoint Phosphate Buffer System In Kidney To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain bases that. The. Phosphate Buffer System In Kidney.
From ar.inspiredpencil.com
Renal Buffer System Phosphate Buffer System In Kidney Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Disturbances of these mechanisms can have serious clinical consequences. All of these contain bases that. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. The principal buffer system is the bicarbonate. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Phosphate Buffer System In Kidney The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. All of these contain bases that.. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Acid, Base, Electrolytes PowerPoint Presentation, free download Phosphate Buffer System In Kidney Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration. Phosphate Buffer System In Kidney.
From open.oregonstate.education
25.5 Physiology of Urine Formation Tubular Reabsorption and Secretion Phosphate Buffer System In Kidney All of these contain bases that. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Azin Nowrouzi, PhD PowerPoint Presentation, free download ID Phosphate Buffer System In Kidney Disturbances of these mechanisms can have serious clinical consequences. The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+].. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT CONTROL OF EXTRACELLULAR FLUID VOLUME AND REGULATION OF RENAL Phosphate Buffer System In Kidney The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID Phosphate Buffer System In Kidney Disturbances of these mechanisms can have serious clinical consequences. All of these contain bases that. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Buffer systems PowerPoint Presentation, free download ID9427698 Phosphate Buffer System In Kidney Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. The kidneys are slower to compensate than the lungs, but renal physiology. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Fluid & Electrolyte Balance PowerPoint Presentation, free Phosphate Buffer System In Kidney Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. All of these contain bases that. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Disturbances of these mechanisms can. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation Phosphate Buffer System In Kidney Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. All of these contain bases that. Disturbances of these mechanisms can have serious clinical consequences. The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the. Phosphate Buffer System In Kidney.
From biosiva.50webs.org
Respiratory System Phosphate Buffer System In Kidney Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. Disturbances of. Phosphate Buffer System In Kidney.
From ar.inspiredpencil.com
Renal Buffer System Phosphate Buffer System In Kidney Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of 7.43 to 7.37, where ph = −log [h+]. Other buffer systems in the human body include the phosphate buffer. Phosphate Buffer System In Kidney.
From philschatz.com
AcidBase Balance · Anatomy and Physiology Phosphate Buffer System In Kidney The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. Other buffer systems in the human body include the phosphate buffer system, proteins,. Phosphate Buffer System In Kidney.
From slideplayer.com
Renal Handling of H+ concentration ppt download Phosphate Buffer System In Kidney All of these contain bases that. Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the.. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Renal Control of AcidBase Balance PowerPoint Presentation, free Phosphate Buffer System In Kidney The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. All of these contain bases that. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Phosphate Buffer System In Kidney The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. Disturbances of these mechanisms can have serious clinical consequences. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a. Phosphate Buffer System In Kidney.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer System In Kidney All of these contain bases that. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. Other buffer systems in the human body include the phosphate buffer. Phosphate Buffer System In Kidney.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer System In Kidney Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. Disturbances of these mechanisms can have serious clinical consequences. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow. Phosphate Buffer System In Kidney.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation Phosphate Buffer System In Kidney The kidneys are slower to compensate than the lungs, but renal physiology has several powerful mechanisms to control ph by the excretion of. The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. Other buffer systems in. Phosphate Buffer System In Kidney.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer System In Kidney Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. Disturbances of these mechanisms can have serious clinical consequences. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to. Phosphate Buffer System In Kidney.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Phosphate Buffer System In Kidney Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. All of these contain bases that. The principal buffer system is the bicarbonate buffer system, mediated through carbonic anhydrase. To maintain cellular function, the body has elaborate mechanisms that maintain blood h+ concentration within a narrow range—typically 37 to 43 neq/l (37 to 43 nmol/l) with a ph of. Phosphate Buffer System In Kidney.
From www.zuniv.net
New Human Physiology Ch 17 Phosphate Buffer System In Kidney Ideally, h+ is 40 neq/l (40 nmol/l) and ph = 7.40. In the nephron, the proximal convoluted tubule cells are able to reabsorb the bicarbonate hco3− ions, and cells in the. Bicarbonate is the predominant extracellular buffer against the fixed acids and it important that its plasma concentration should be defended against renal loss. The principal buffer system is the. Phosphate Buffer System In Kidney.