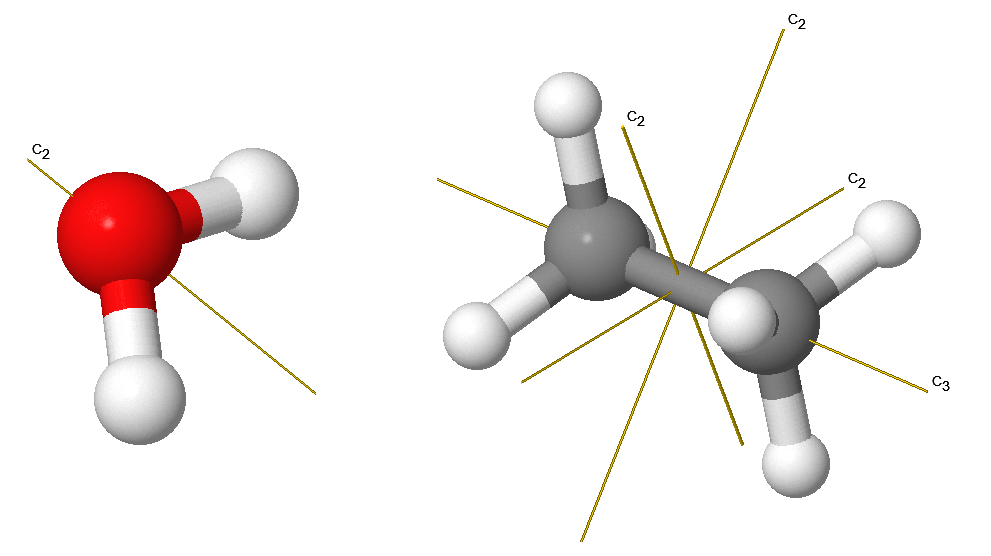
Which Orbitals Have Centre Of Symmetry . The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. The action of this operator, denoted by the symbol i , is to replace the x ,. Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. For example, consider transition metals’ d orbitals. In tetrahedral complexes, which do not have. The fifth d orbital is shaped like. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. Much of this chapter is devoted to how this can be carried out in a. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator.
from chem.libretexts.org
Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. For example, consider transition metals’ d orbitals. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. In tetrahedral complexes, which do not have. Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. The action of this operator, denoted by the symbol i , is to replace the x ,. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. The fifth d orbital is shaped like. Much of this chapter is devoted to how this can be carried out in a.
12.2 Symmetry Elements Chemistry LibreTexts
Which Orbitals Have Centre Of Symmetry Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. In tetrahedral complexes, which do not have. The action of this operator, denoted by the symbol i , is to replace the x ,. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. For example, consider transition metals’ d orbitals. The fifth d orbital is shaped like. Much of this chapter is devoted to how this can be carried out in a. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig.
From www.fluxsci.com
8 Drawing Molecular Orbital Diagrams — Flux Science Which Orbitals Have Centre Of Symmetry The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. The action of this operator, denoted by the symbol i , is to replace the x ,. The fifth d orbital is shaped like. Symmetry can be used to characterize the core,. Which Orbitals Have Centre Of Symmetry.
From www.researchgate.net
Symmetryadapted molecular orbitals containing the metallic valence Which Orbitals Have Centre Of Symmetry Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. Much of this chapter is devoted to how this can be carried out in a. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. The p x and p y orbitals have π symmetry (nodal. Which Orbitals Have Centre Of Symmetry.
From www.youtube.com
L10 Symmetry Applied to 3D Orbitals YouTube Which Orbitals Have Centre Of Symmetry Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. Much of this chapter is devoted to how this can be carried out in a. For example, consider transition metals’ d. Which Orbitals Have Centre Of Symmetry.
From www.slideserve.com
PPT Lecture 37 Symmetry Orbitals The material in this lecture covers Which Orbitals Have Centre Of Symmetry Much of this chapter is devoted to how this can be carried out in a. The fifth d orbital is shaped like. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. Symmetry can be used to characterize the core, bonding, nonbonding, and. Which Orbitals Have Centre Of Symmetry.
From www.slideserve.com
PPT Lecture 14 APPLICATIONS OF GROUP THEORY 1) Symmetry of atomic Which Orbitals Have Centre Of Symmetry The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. In tetrahedral complexes, which do not have. Much of this chapter is devoted to. Which Orbitals Have Centre Of Symmetry.
From chem.libretexts.org
Introduction to Crystal Field Theory Chemistry LibreTexts Which Orbitals Have Centre Of Symmetry In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. The fifth d orbital is shaped like. Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. For example, consider transition metals’ d orbitals. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding. Which Orbitals Have Centre Of Symmetry.
From socratic.org
Which are the orbitals(s,p,d,f) have center of symmetry? Socratic Which Orbitals Have Centre Of Symmetry The fifth d orbital is shaped like. In tetrahedral complexes, which do not have. The action of this operator, denoted by the symbol i , is to replace the x ,. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called. Which Orbitals Have Centre Of Symmetry.
From www.researchgate.net
(a) CASSCF d orbitals on the Mn4 center of S B 3. Symmetry labels Which Orbitals Have Centre Of Symmetry For example, consider transition metals’ d orbitals. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. The action of this operator, denoted by the symbol i , is to replace the x ,. Much of this chapter is devoted to how this can be carried out in a. The fifth d orbital is shaped like.. Which Orbitals Have Centre Of Symmetry.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills Which Orbitals Have Centre Of Symmetry For example, consider transition metals’ d orbitals. The fifth d orbital is shaped like. The action of this operator, denoted by the symbol i , is to replace the x ,. Much of this chapter is devoted to how this can be carried out in a. In tetrahedral complexes, which do not have. Symmetry can be used to characterize the. Which Orbitals Have Centre Of Symmetry.
From www.numerade.com
SOLVED Sort the images below based on the symmetry of the molecular Which Orbitals Have Centre Of Symmetry Much of this chapter is devoted to how this can be carried out in a. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. The action of this operator, denoted by the symbol i , is to replace the x ,. In tetrahedral complexes, which do not have. A homonuclear diatomic molecule possesses a centre. Which Orbitals Have Centre Of Symmetry.
From www.slideserve.com
PPT Character Tables for Point Groups PowerPoint Presentation, free Which Orbitals Have Centre Of Symmetry In tetrahedral complexes, which do not have. Much of this chapter is devoted to how this can be carried out in a. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. For. Which Orbitals Have Centre Of Symmetry.
From www.stereoelectronics.org
Selection rules and orbital symmetry Which Orbitals Have Centre Of Symmetry The action of this operator, denoted by the symbol i , is to replace the x ,. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. In tetrahedral complexes, which do not have. For example, consider transition metals’ d orbitals. The fifth d orbital is shaped like. Proper molecular orbitals are. Which Orbitals Have Centre Of Symmetry.
From chemistry.stackexchange.com
chemistry What is symmetry of p and dorbitals in {D_{3h Which Orbitals Have Centre Of Symmetry In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. The fifth d orbital is shaped like. Much of this chapter is devoted to how this can be carried out in a. Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. The p x and. Which Orbitals Have Centre Of Symmetry.
From www.coursehero.com
What is the symmetry of each of the f orbitals in the D2h point Which Orbitals Have Centre Of Symmetry The action of this operator, denoted by the symbol i , is to replace the x ,. The fifth d orbital is shaped like. Much of this chapter is devoted to how this can be carried out in a. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. For example, consider transition metals’ d orbitals. In tetrahedral complexes, which do. Which Orbitals Have Centre Of Symmetry.
From www.vedantu.com
Define an atomic orbital. Which Orbitals Have Centre Of Symmetry Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. The fifth d orbital is shaped like. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. Much of this chapter is devoted to how this can be carried out in a. The action of this operator, denoted. Which Orbitals Have Centre Of Symmetry.
From www.chemtube3d.com
Metal d orbitals in an octahedral crystal field Which Orbitals Have Centre Of Symmetry For example, consider transition metals’ d orbitals. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for.. Which Orbitals Have Centre Of Symmetry.
From www.technocrazed.com
Orbitals (s) Three fold symmetry. (p) Shown px, one of three possible Which Orbitals Have Centre Of Symmetry In tetrahedral complexes, which do not have. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. For example, consider transition metals’ d orbitals. The fifth d orbital is. Which Orbitals Have Centre Of Symmetry.
From www.youtube.com
Gerade and Ungerade Molecular Orbitals. (SYMMETRY OF MOLECULAR ORBITALS Which Orbitals Have Centre Of Symmetry Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. In tetrahedral complexes, which do not have. The action of this operator, denoted by the symbol i , is. Which Orbitals Have Centre Of Symmetry.
From www.slideserve.com
PPT Lecture 37 Symmetry Orbitals The material in this lecture covers Which Orbitals Have Centre Of Symmetry A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. In tetrahedral complexes, which do not have. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. For example, consider transition metals’ d orbitals.. Which Orbitals Have Centre Of Symmetry.
From www.slideserve.com
PPT Lecture 14 APPLICATIONS OF GROUP THEORY 1) Symmetry of atomic Which Orbitals Have Centre Of Symmetry The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. The fifth d orbital is shaped like. A homonuclear diatomic molecule possesses a centre of symmetry and the. Which Orbitals Have Centre Of Symmetry.
From www.youtube.com
Explain the elements of symmetry giving examples Stereochemistry Which Orbitals Have Centre Of Symmetry Much of this chapter is devoted to how this can be carried out in a. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. In tetrahedral complexes, which do not have. The p x and p y orbitals have π symmetry (nodal plane containing the bonding. Which Orbitals Have Centre Of Symmetry.
From www.numerade.com
SOLVED Label the following orbitals as either gerade symmetry Or Which Orbitals Have Centre Of Symmetry Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. The fifth d orbital is shaped like. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled. Which Orbitals Have Centre Of Symmetry.
From unacademy.com
Concept of shapes of s, p & d orbitals Which Orbitals Have Centre Of Symmetry A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. The fifth d orbital is shaped like. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry. Which Orbitals Have Centre Of Symmetry.
From www.researchgate.net
Molecular orbital interaction diagram for Re[Cl(CO2)] of Cs symmetry Which Orbitals Have Centre Of Symmetry For example, consider transition metals’ d orbitals. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$. Which Orbitals Have Centre Of Symmetry.
From www.numerade.com
SOLVED Label the following orbitals as either gerade symmetry or Which Orbitals Have Centre Of Symmetry Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. Much of this chapter is devoted to how this can be carried out in a. The p x and p y orbitals have π symmetry (nodal plane containing. Which Orbitals Have Centre Of Symmetry.
From www.britannica.com
Centre of symmetry physics Britannica Which Orbitals Have Centre Of Symmetry The action of this operator, denoted by the symbol i , is to replace the x ,. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. In tetrahedral complexes, which do not have. The fifth d orbital is shaped like. For. Which Orbitals Have Centre Of Symmetry.
From www.fluxsci.com
8 Drawing Molecular Orbital Diagrams — Flux Science Which Orbitals Have Centre Of Symmetry Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level. Which Orbitals Have Centre Of Symmetry.
From courses.lumenlearning.com
5.5 Hybrid Atomic Orbitals General College Chemistry I Which Orbitals Have Centre Of Symmetry Proper molecular orbitals are influenced by all the nuclei in a molecule, and require consideration of the full structure and symmetry of a molecule for. Much of this chapter is devoted to how this can be carried out in a. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. For example, consider transition metals’ d orbitals. The action of this. Which Orbitals Have Centre Of Symmetry.
From www.slideserve.com
PPT Lecture 14 APPLICATIONS OF GROUP THEORY 1) Symmetry of atomic Which Orbitals Have Centre Of Symmetry Much of this chapter is devoted to how this can be carried out in a. For example, consider transition metals’ d orbitals. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. The fifth d orbital is shaped like. Proper molecular orbitals. Which Orbitals Have Centre Of Symmetry.
From chemistrytalk.org
Electron Orbitals & Orbital Shapes ChemTalk Which Orbitals Have Centre Of Symmetry In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. In tetrahedral complexes, which do not have. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. The action of this operator, denoted by the symbol i , is. Which Orbitals Have Centre Of Symmetry.
From chem.libretexts.org
Radial and Angular Parts of Atomic Orbitals Chemistry LibreTexts Which Orbitals Have Centre Of Symmetry In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. Much of this chapter is devoted to how this can be carried out in a. The action of this operator, denoted by the symbol i , is to replace the x ,. In tetrahedral complexes, which do not have. The p x and p y orbitals have π symmetry (nodal plane. Which Orbitals Have Centre Of Symmetry.
From www.britannica.com
Orbital Chemistry, Physics & Applications Britannica Which Orbitals Have Centre Of Symmetry In tetrahedral complexes, which do not have. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. Much of this chapter is devoted to how this can be carried out in a. A homonuclear diatomic molecule possesses a centre of symmetry and. Which Orbitals Have Centre Of Symmetry.
From circuitlibbottega.z21.web.core.windows.net
Orbital Diagram For Si Which Orbitals Have Centre Of Symmetry The fifth d orbital is shaped like. Much of this chapter is devoted to how this can be carried out in a. In tetrahedral complexes, which do not have. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion. Which Orbitals Have Centre Of Symmetry.
From chem.libretexts.org
12.2 Symmetry Elements Chemistry LibreTexts Which Orbitals Have Centre Of Symmetry A homonuclear diatomic molecule possesses a centre of symmetry and the corresponding operator is called the inversion operator. The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals.. Which Orbitals Have Centre Of Symmetry.
From www.slideserve.com
PPT Character Tables for Point Groups PowerPoint Presentation, free Which Orbitals Have Centre Of Symmetry The p x and p y orbitals have π symmetry (nodal plane containing the bonding axis) and are labeled π nb in the mo energy level diagram, fig. In octahedral complexes, they are labelled $\mathrm{t_{2g}}$ and $\mathrm{e_g}$. Symmetry can be used to characterize the core, bonding, nonbonding, and antibonding molecular orbitals. For example, consider transition metals’ d orbitals. Proper molecular. Which Orbitals Have Centre Of Symmetry.