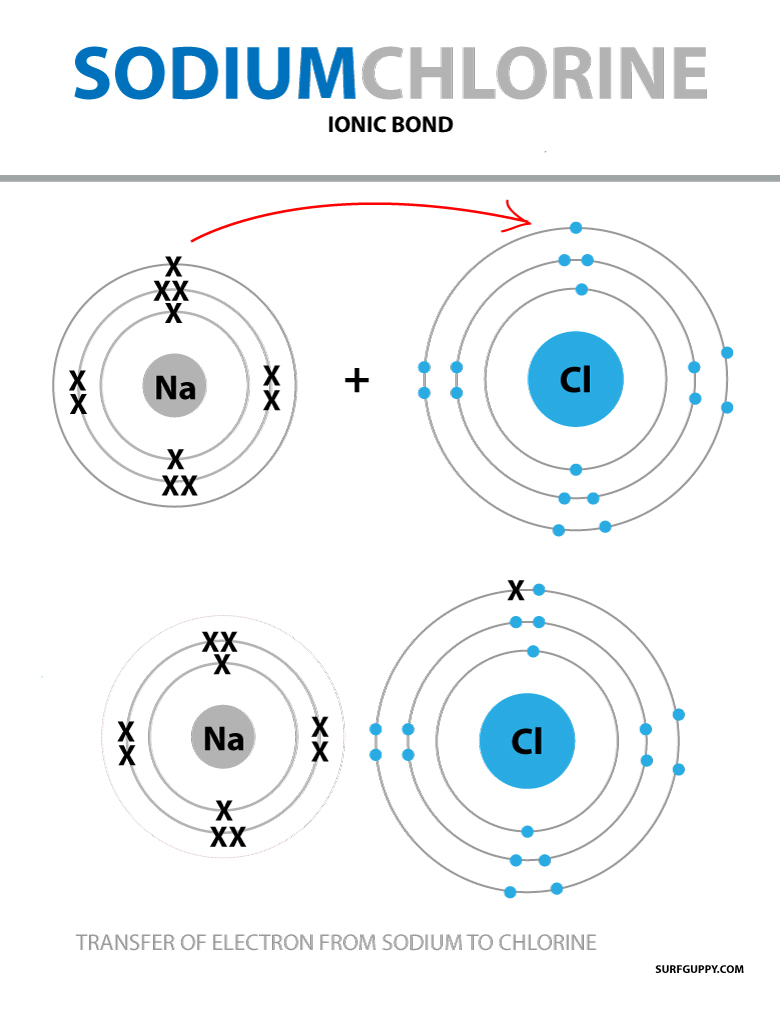
Model Of An Ionic Bond . An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. The transfer results in the atom that loses. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Opposite charges attract and like charges repel. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. These oppositely charged ions attract each other to form ionic networks (or lattices). Learn more about ionic bonds in this article. Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely. Electrostatics explains why this happens: Infographic poster, fact sheet and ionic bonding mats resource. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process.
from surfguppy.com
These oppositely charged ions attract each other to form ionic networks (or lattices). Opposite charges attract and like charges repel. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. The transfer results in the atom that loses. Learn more about ionic bonds in this article. Electrostatics explains why this happens: Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound.
What is Ionic Bond Surfguppy Chemistry made easy visual learning
Model Of An Ionic Bond Electrostatics explains why this happens: These oppositely charged ions attract each other to form ionic networks (or lattices). The transfer results in the atom that loses. Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Learn more about ionic bonds in this article. An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Infographic poster, fact sheet and ionic bonding mats resource. Opposite charges attract and like charges repel. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Electrostatics explains why this happens:
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID4493576 Model Of An Ionic Bond Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. Learn more about ionic bonds in this article. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Ionic bonding is. Model Of An Ionic Bond.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Model Of An Ionic Bond Learn more about ionic bonds in this article. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. A dot and. Model Of An Ionic Bond.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Model Of An Ionic Bond Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. These oppositely charged ions attract each other to form ionic networks (or lattices). Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Ionic bonding is the complete transfer of valence electron(s) between atoms. Model Of An Ionic Bond.
From www.trentu.ca
PreChemistry Model Of An Ionic Bond Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Infographic poster, fact sheet and ionic bonding mats resource. Opposite charges attract and like charges repel. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Electrostatics explains why this happens: Ionic. Model Of An Ionic Bond.
From stock.adobe.com
Ionic bond and electrostatic attraction from chemical bonding outline Model Of An Ionic Bond An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. These oppositely charged ions attract each other to form ionic networks (or lattices). Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Learn more about ionic bonds in this article. Infographic poster,. Model Of An Ionic Bond.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Model Of An Ionic Bond Opposite charges attract and like charges repel. Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Such a bond forms when the valence (outermost) electrons of one atom. Model Of An Ionic Bond.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Model Of An Ionic Bond These oppositely charged ions attract each other to form ionic networks (or lattices). A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An ionic bond or electrovalent bond is an electrostatic attraction. Model Of An Ionic Bond.
From www.youtube.com
Ionic Bonding Part 3 YouTube Model Of An Ionic Bond Electrostatics explains why this happens: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to. Model Of An Ionic Bond.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Model Of An Ionic Bond Learn more about ionic bonds in this article. The transfer results in the atom that loses. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. Compounds composed of ions are called ionic compounds (or salts), and their constituent. Model Of An Ionic Bond.
From www.sliderbase.com
Chemical Bonds Model Of An Ionic Bond Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Compounds composed of ions are called ionic compounds (or salts),. Model Of An Ionic Bond.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Model Of An Ionic Bond Learn more about ionic bonds in this article. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Opposite charges attract and like charges repel. Ionic bond, type of linkage formed from the. Model Of An Ionic Bond.
From depositphotos.com
Structure Ionic Bond White Background Isolated Vector Illustration Model Of An Ionic Bond Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. Electrostatics explains why this happens: An ionic bond or electrovalent bond. Model Of An Ionic Bond.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary Model Of An Ionic Bond Infographic poster, fact sheet and ionic bonding mats resource. Opposite charges attract and like charges repel. The transfer results in the atom that loses. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Electrostatics explains why this happens: Compounds composed of ions are called ionic compounds (or salts), and their constituent. Model Of An Ionic Bond.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Model Of An Ionic Bond Learn more about ionic bonds in this article. An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently. Model Of An Ionic Bond.
From www.carlsonstockart.com
Ionic Bonding Carlson Stock Art Model Of An Ionic Bond Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Infographic poster, fact sheet and ionic bonding mats resource. The transfer results in the atom that loses. Learn more about ionic bonds in this article. Opposite charges attract and like charges repel. Ionic bonding is the complete transfer of valence. Model Of An Ionic Bond.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Model Of An Ionic Bond Learn more about ionic bonds in this article. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Infographic poster, fact sheet and ionic bonding mats resource. The transfer results in the atom that loses. Opposite charges attract and like charges repel. Ionic bonding is the complete transfer of valence electron(s) between. Model Of An Ionic Bond.
From chemizi.blogspot.com
Ionic bonddefinitionexampleproperties and formation condition Model Of An Ionic Bond Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An ionic bond or electrovalent bond is an electrostatic attraction where. Model Of An Ionic Bond.
From stock.adobe.com
Ionic covalent bonds examples. Chemical structural models. Atoms Model Of An Ionic Bond Opposite charges attract and like charges repel. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Infographic poster, fact sheet and ionic bonding mats resource. A dot and cross diagram is one. Model Of An Ionic Bond.
From en.wikipedia.org
Ionic bonding Wikipedia Model Of An Ionic Bond Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Opposite charges attract and like charges repel. These oppositely charged ions attract each other to form ionic networks (or lattices). An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Electrostatics. Model Of An Ionic Bond.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Model Of An Ionic Bond Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Learn more about ionic bonds in this article. An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Infographic poster, fact sheet and ionic bonding mats resource. Ionic bonding is the complete transfer. Model Of An Ionic Bond.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Model Of An Ionic Bond Learn more about ionic bonds in this article. Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely. The transfer results in the atom that loses. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Compounds composed of ions. Model Of An Ionic Bond.
From www.youtube.com
Understanding the Ionic Bonding Model YouTube Model Of An Ionic Bond The transfer results in the atom that loses. These oppositely charged ions attract each other to form ionic networks (or lattices). An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. Model Of An Ionic Bond.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts Model Of An Ionic Bond Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Learn more about ionic bonds in this article. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. Ionic bonding is. Model Of An Ionic Bond.
From sciencenotes.org
Ionic Bond Definition and Examples Model Of An Ionic Bond The transfer results in the atom that loses. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Learn more about ionic bonds in this article. Electrostatics explains why this happens: These oppositely charged ions attract each other to form ionic networks (or lattices). A dot and cross diagram is. Model Of An Ionic Bond.
From chemistry.about.com
Examples of Ionic Bonds and Ionic Compounds Model Of An Ionic Bond Infographic poster, fact sheet and ionic bonding mats resource. Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Electrostatics explains why this happens: These oppositely charged ions attract each other to form. Model Of An Ionic Bond.
From www.dreamstime.com
Ionic Bond Vector Illustration. Labeled Diagram with Formation Model Of An Ionic Bond Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. The transfer results in the atom that loses. Opposite charges attract and like charges repel. A dot and cross diagram is one way to model the transfer of electrons. Model Of An Ionic Bond.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Model Of An Ionic Bond Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Electrostatics explains why this happens: Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. Compounds composed of ions are called. Model Of An Ionic Bond.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Model Of An Ionic Bond Electrostatics explains why this happens: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Learn more about ionic bonds in this article. Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely. Ionic bonding models are generally. Model Of An Ionic Bond.
From examples.yourdictionary.com
Ionic Bond Examples YourDictionary Model Of An Ionic Bond A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. The transfer results in the atom that loses. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. An ionic. Model Of An Ionic Bond.
From commons.wikimedia.org
FileIonic bonding.svg Wikimedia Commons Model Of An Ionic Bond Electrostatics explains why this happens: An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are. Ionic bonding is the complete. Model Of An Ionic Bond.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Model Of An Ionic Bond Infographic poster, fact sheet and ionic bonding mats resource. An ionic bond or electrovalent bond is an electrostatic attraction where one atom donates an electron to another atom. Learn more about ionic bonds in this article. Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely. Opposite charges. Model Of An Ionic Bond.
From www2.victoriacollege.edu
formation of ionic bonds Model Of An Ionic Bond These oppositely charged ions attract each other to form ionic networks (or lattices). Electrostatics explains why this happens: A dot and cross diagram is one way to model the transfer of electrons that occurs during this process. Infographic poster, fact sheet and ionic bonding mats resource. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions. Model Of An Ionic Bond.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Model Of An Ionic Bond Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely.. Model Of An Ionic Bond.
From www.chemistrylearner.com
Ionic Bond Facts, Definition, Properties, Examples, & Diagrams Model Of An Ionic Bond Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Opposite charges attract and like charges repel. Ionic bonding is the complete transfer of valence electron(s) between atoms and is a type of chemical bond that generates two oppositely. Electrostatics explains why this happens: The transfer results in the atom that loses.. Model Of An Ionic Bond.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Model Of An Ionic Bond Opposite charges attract and like charges repel. These oppositely charged ions attract each other to form ionic networks (or lattices). Learn more about ionic bonds in this article. The transfer results in the atom that loses. Infographic poster, fact sheet and ionic bonding mats resource. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are. Model Of An Ionic Bond.