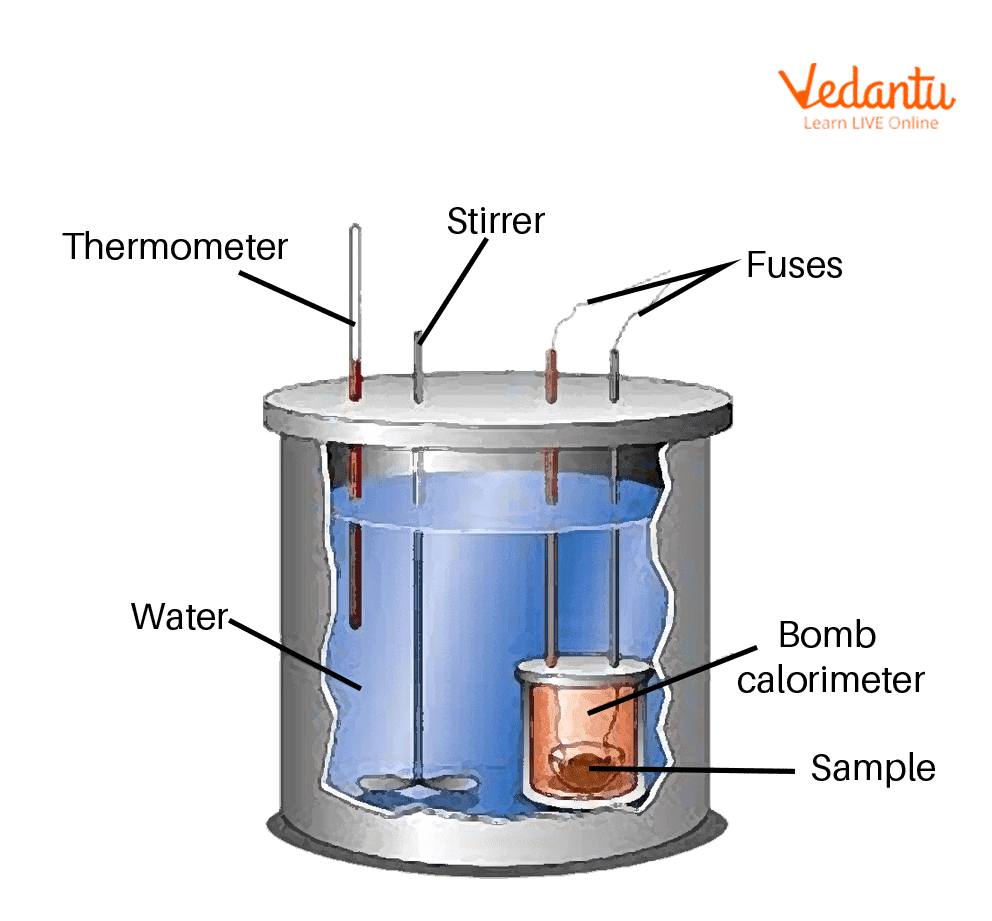
Calorimetry Experiment Igcse . Energy content is the amount of heat produced by the burning. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Measuring heat transfers is called calorimetry. The diagram shows a simple calorimetry experiment to measure the heat energy. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. We do this using simple calorimetry. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Investigate temperature changes accompanying some of the following types of change: We can experimentally determine the relative amounts of energy released by a fuel.
from www.vedantu.com
Measuring heat transfers is called calorimetry. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples The diagram shows a simple calorimetry experiment to measure the heat energy. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Energy content is the amount of heat produced by the burning. We do this using simple calorimetry. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Investigate temperature changes accompanying some of the following types of change: We can experimentally determine the relative amounts of energy released by a fuel.
Bomb Calorimeter Learn Important Terms and Concepts
Calorimetry Experiment Igcse 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. We do this using simple calorimetry. The diagram shows a simple calorimetry experiment to measure the heat energy. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples We can experimentally determine the relative amounts of energy released by a fuel. Investigate temperature changes accompanying some of the following types of change: 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Energy content is the amount of heat produced by the burning. Measuring heat transfers is called calorimetry.
From studylib.net
experiment 9 Thermochemistry Calorimetry Calorimetry Experiment Igcse Investigate temperature changes accompanying some of the following types of change: The diagram shows a simple calorimetry experiment to measure the heat energy. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. We can experimentally determine the relative amounts of energy released by a fuel. Different food samples can be burned in a simple calorimetry. Calorimetry Experiment Igcse.
From www.studypool.com
SOLUTION Calorimetry Biology Revision with Questions (GCSE Edexcel Calorimetry Experiment Igcse The diagram shows a simple calorimetry experiment to measure the heat energy. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Measuring heat transfers is called calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and. Calorimetry Experiment Igcse.
From ar.inspiredpencil.com
Calorimeter Calorimetry Experiment Igcse 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples We can experimentally determine the relative amounts of energy released by a fuel.. Calorimetry Experiment Igcse.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimetry Experiment Igcse Measuring heat transfers is called calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. Energy content is the amount of heat produced by the burning. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. The diagram shows a simple calorimetry experiment to measure the heat energy. Different food samples can. Calorimetry Experiment Igcse.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimetry Experiment Igcse Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Energy content is the amount of heat produced by the burning. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation.. Calorimetry Experiment Igcse.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Experiment Igcse The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Investigate temperature changes accompanying some of the following types of change: We do this using simple calorimetry. Measuring heat transfers is called calorimetry. The diagram shows a simple calorimetry experiment to measure the heat energy. Different food samples can be burned in a simple. Calorimetry Experiment Igcse.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Calorimetry Experiment Igcse The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Measuring heat transfers is called calorimetry. The diagram shows a simple calorimetry experiment to measure the heat energy. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving. Calorimetry Experiment Igcse.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Calorimetry Experiment Igcse 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Measuring heat transfers is called calorimetry. Investigate temperature changes accompanying some of the following types of. Calorimetry Experiment Igcse.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.2 Calorimetry翰林国际教育 Calorimetry Experiment Igcse The diagram shows a simple calorimetry experiment to measure the heat energy. Energy content is the amount of heat produced by the burning. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. 3:02 describe simple calorimetry experiments for reactions. Calorimetry Experiment Igcse.
From users.highland.edu
Calorimetry Calorimetry Experiment Igcse We can experimentally determine the relative amounts of energy released by a fuel. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Energy content is the amount of heat produced by the burning. We do this using simple calorimetry. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement,. Calorimetry Experiment Igcse.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Experiment Igcse We do this using simple calorimetry. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Measuring heat transfers is called calorimetry. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. The diagram shows a simple calorimetry experiment to measure the heat energy. Energy content is the amount of heat produced. Calorimetry Experiment Igcse.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Calorimetry Experiment Igcse We can experimentally determine the relative amounts of energy released by a fuel. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Measuring heat transfers is called calorimetry. 3.2 describe simple calorimetry experiments for reactions such. Calorimetry Experiment Igcse.
From www.youtube.com
Calorimetry Experiment YouTube Calorimetry Experiment Igcse Energy content is the amount of heat produced by the burning. Measuring heat transfers is called calorimetry. The diagram shows a simple calorimetry experiment to measure the heat energy. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. We do this using simple calorimetry. Investigate temperature changes accompanying some of the following types of change:. Calorimetry Experiment Igcse.
From www.youtube.com
7. Edexcel GCSE Biology Calorimetry YouTube Calorimetry Experiment Igcse 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. We do this using simple calorimetry. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Energy content is the amount of heat produced by the burning. We can experimentally determine the relative amounts of energy released by a fuel. The energy. Calorimetry Experiment Igcse.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimetry Experiment Igcse The diagram shows a simple calorimetry experiment to measure the heat energy. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. We do this using simple calorimetry. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples The energy your body needs for living (running, breathing,. Calorimetry Experiment Igcse.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Experiment Igcse 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. We can experimentally determine the relative amounts of energy released by a fuel. Energy content is the amount of heat produced by the burning. We do this using simple calorimetry. Investigate temperature changes accompanying some of the following types of change: Different food samples can be. Calorimetry Experiment Igcse.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Experiment Igcse 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Investigate temperature changes accompanying some of the following types of change: Measuring heat transfers is called calorimetry. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and. Calorimetry Experiment Igcse.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Experiment Igcse 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Investigate temperature changes accompanying some of the following types of change: The diagram shows a simple calorimetry experiment to measure the heat energy. We do this using simple calorimetry. Energy content is the amount of heat produced by the burning. Measuring heat transfers is called calorimetry.. Calorimetry Experiment Igcse.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Calorimetry Experiment Igcse 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Measuring heat transfers is called calorimetry. We can experimentally determine the relative amounts of energy released by a fuel. The diagram shows a simple calorimetry experiment to measure the heat energy. The energy your body needs for living (running, breathing, thinking) comes from the food you. Calorimetry Experiment Igcse.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Experiment Igcse The energy your body needs for living (running, breathing, thinking) comes from the food you eat. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Investigate temperature changes accompanying some of the following types of change: We can experimentally determine the relative amounts of energy released by a fuel. 3:02 describe simple calorimetry experiments for. Calorimetry Experiment Igcse.
From www.youtube.com
INVESTIGATING ENERGY CONTENT IN FOOD SIMPLE CALORIMETRY IGCSE Calorimetry Experiment Igcse 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Energy content is the amount of heat produced by the burning. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Investigate temperature changes accompanying some. Calorimetry Experiment Igcse.
From www.studocu.com
Calorimetry Experiment 25 Calorimetry To determine the specific heat Calorimetry Experiment Igcse Measuring heat transfers is called calorimetry. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. The diagram shows a simple calorimetry experiment to measure the heat energy. Investigate temperature changes accompanying some of the following types of change: We do this using simple calorimetry. Energy content is the amount of heat produced by. Calorimetry Experiment Igcse.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Experiment Igcse Measuring heat transfers is called calorimetry. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples The diagram shows a simple calorimetry experiment to measure the heat energy. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. We can experimentally determine the relative amounts. Calorimetry Experiment Igcse.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Calorimetry Experiment Igcse 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples The diagram shows a simple calorimetry experiment to measure the heat energy. We can experimentally determine the relative amounts of energy released by a fuel. Investigate temperature. Calorimetry Experiment Igcse.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Experiment Igcse The diagram shows a simple calorimetry experiment to measure the heat energy. Energy content is the amount of heat produced by the burning. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. 3:02 describe simple calorimetry. Calorimetry Experiment Igcse.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.6 Practical Investigating Temperature Calorimetry Experiment Igcse Investigate temperature changes accompanying some of the following types of change: Measuring heat transfers is called calorimetry. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. We do this using simple calorimetry. The energy your body needs for living (running, breathing,. Calorimetry Experiment Igcse.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Experiment Igcse 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Investigate temperature changes accompanying some of the following types of change: Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples. Calorimetry Experiment Igcse.
From www.scribd.com
Calorimetry Experiment PDF Heat Enthalpy Calorimetry Experiment Igcse The energy your body needs for living (running, breathing, thinking) comes from the food you eat. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. We can experimentally determine the relative amounts of energy released by a fuel. Different food samples. Calorimetry Experiment Igcse.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Experiment Igcse Energy content is the amount of heat produced by the burning. Investigate temperature changes accompanying some of the following types of change: 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Measuring heat transfers is called calorimetry. The diagram shows a simple calorimetry experiment to measure the heat energy. 3.2 describe simple calorimetry experiments for. Calorimetry Experiment Igcse.
From www.baamboozle.com
Heat Gain and Heat Loss Baamboozle Baamboozle The Most Fun Calorimetry Experiment Igcse The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Energy content is the amount of heat produced by the burning. We do this using simple calorimetry. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Measuring heat transfers is called calorimetry. 3:02 describe simple calorimetry experiments for reactions. Calorimetry Experiment Igcse.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement Calorimetry Experiment Igcse 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Measuring heat transfers is called calorimetry. Energy content is the amount of heat produced by the burning. Investigate temperature changes accompanying some of the following types of change: The energy your body needs for living (running, breathing, thinking) comes from the food you eat. We can. Calorimetry Experiment Igcse.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Experiment Igcse Measuring heat transfers is called calorimetry. The diagram shows a simple calorimetry experiment to measure the heat energy. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. We do this using simple calorimetry. Different food samples can be burned. Calorimetry Experiment Igcse.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Experiment Igcse Energy content is the amount of heat produced by the burning. Measuring heat transfers is called calorimetry. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. Different food samples can be burned in a simple calorimetry experiment to compare the energy contents of the samples Investigate temperature changes accompanying some of the following types of. Calorimetry Experiment Igcse.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Experiment Igcse We can experimentally determine the relative amounts of energy released by a fuel. We do this using simple calorimetry. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. Measuring heat transfers is called calorimetry. 3.2 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralization. Different food samples can be. Calorimetry Experiment Igcse.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Calorimetry Experiment Igcse We can experimentally determine the relative amounts of energy released by a fuel. The energy your body needs for living (running, breathing, thinking) comes from the food you eat. 3:02 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation. The diagram shows a simple calorimetry experiment to measure the heat energy. Investigate temperature changes accompanying some. Calorimetry Experiment Igcse.