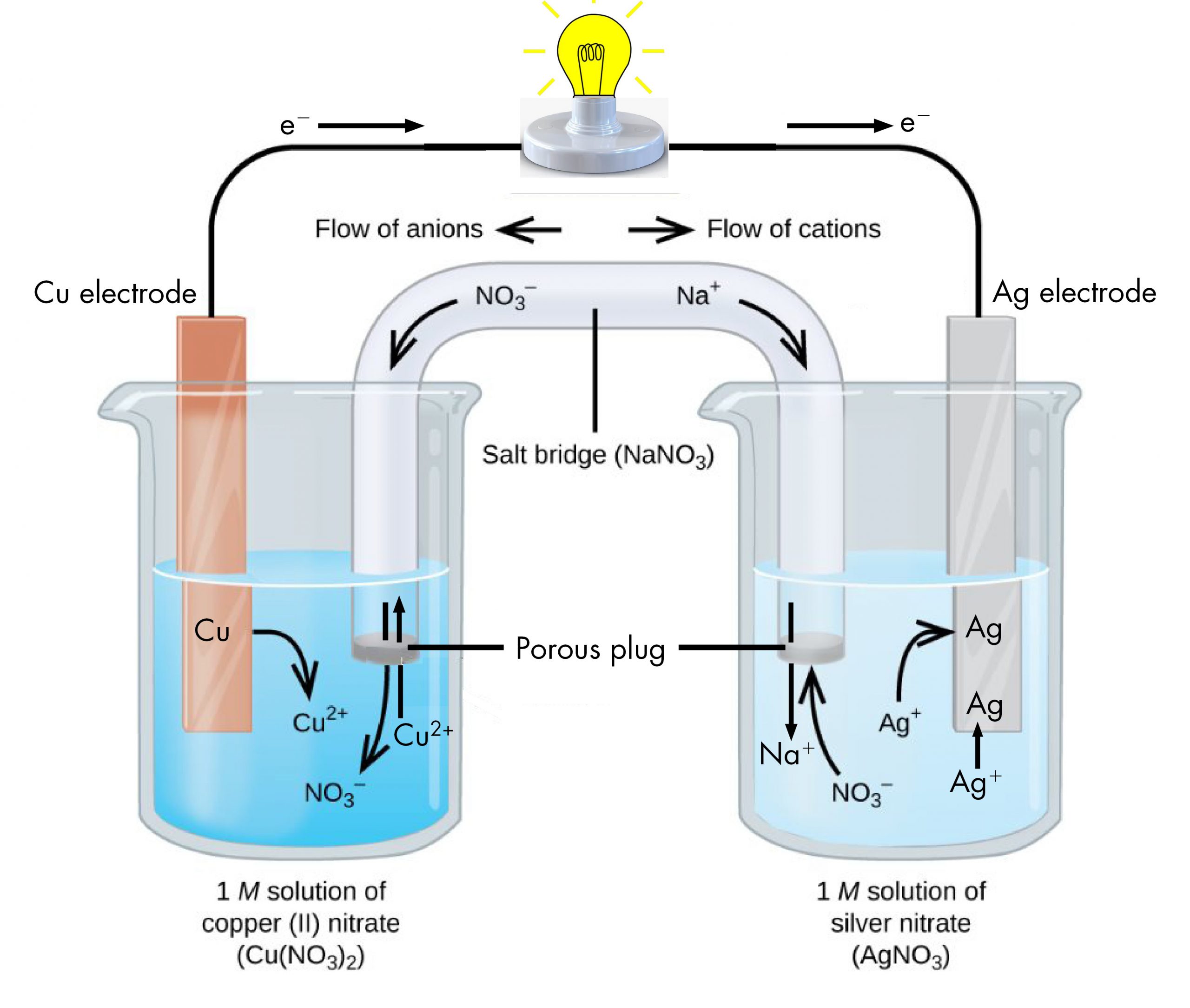
Copper And Zinc Electrochemical Cell . Then a zinc electrode is placed in the zinc sulfate solution. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. A zinc sulfate solution is floated on top of the copper sulfate solution; A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The salt bridge prevents the. The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. In the process of the reaction,. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. For the metallic components (zinc, copper, copper.
from ar.inspiredpencil.com
One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. A zinc sulfate solution is floated on top of the copper sulfate solution; The salt bridge prevents the. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. For the metallic components (zinc, copper, copper. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. In the process of the reaction,.
Copper Electrolytic Cell
Copper And Zinc Electrochemical Cell For the metallic components (zinc, copper, copper. In the process of the reaction,. A zinc sulfate solution is floated on top of the copper sulfate solution; One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The salt bridge prevents the. For the metallic components (zinc, copper, copper. The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. Then a zinc electrode is placed in the zinc sulfate solution. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Copper And Zinc Electrochemical Cell In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. For the metallic components (zinc, copper, copper. A simple. Copper And Zinc Electrochemical Cell.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Copper And Zinc Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. A zinc sulfate solution is floated on top of the copper sulfate solution;. Copper And Zinc Electrochemical Cell.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Copper And Zinc Electrochemical Cell The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. For the metallic components (zinc, copper, copper. In the process of the reaction,. A zinc sulfate solution is. Copper And Zinc Electrochemical Cell.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Copper And Zinc Electrochemical Cell In the process of the reaction,. For the metallic components (zinc, copper, copper. The salt bridge prevents the. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. In. Copper And Zinc Electrochemical Cell.
From www.slideserve.com
PPT What is an Electrochemical Cell? PowerPoint Presentation, free Copper And Zinc Electrochemical Cell A zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The salt bridge prevents the. A simple electrochemical cell can be made. Copper And Zinc Electrochemical Cell.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Copper And Zinc Electrochemical Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The salt bridge prevents the. Then a. Copper And Zinc Electrochemical Cell.
From www.coursehero.com
[Solved] Sketch a diagram of a copper/zinc Daniell cell. Label all the Copper And Zinc Electrochemical Cell The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A simple electrochemical cell can be made from copper. Copper And Zinc Electrochemical Cell.
From www.slideserve.com
PPT Oxidation Reduction (Redox) PowerPoint Presentation, free Copper And Zinc Electrochemical Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. Then a zinc electrode is placed in the zinc sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; The salt bridge prevents the. A typical. Copper And Zinc Electrochemical Cell.
From classnotes.org.in
Extraction of Copper and Zinc Chemistry, Class 12, General Principles Copper And Zinc Electrochemical Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. The processes responsible for the current flow in an electrochemical cell depend on which part. Copper And Zinc Electrochemical Cell.
From www1.chem.umn.edu
CopperZinc Galvanic Cell Copper And Zinc Electrochemical Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Then a zinc electrode is placed in the zinc sulfate solution. In the process of the reaction,. One reactant. Copper And Zinc Electrochemical Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Copper And Zinc Electrochemical Cell One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. For the metallic components (zinc, copper, copper. In the process of the reaction,. A zinc sulfate solution is floated on top of the copper sulfate solution; In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. A. Copper And Zinc Electrochemical Cell.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Copper And Zinc Electrochemical Cell In the process of the reaction,. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Then a zinc electrode is placed in the zinc sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The. Copper And Zinc Electrochemical Cell.
From byjus.com
Daniell Cell Definition, Construction & Working with Cell Reactions Copper And Zinc Electrochemical Cell Then a zinc electrode is placed in the zinc sulfate solution. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. In the process of the reaction,. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each. Copper And Zinc Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2810901 Copper And Zinc Electrochemical Cell A zinc sulfate solution is floated on top of the copper sulfate solution; A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. In a spontaneous reaction electrons leave the zinc, go. Copper And Zinc Electrochemical Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Copper And Zinc Electrochemical Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. The salt bridge prevents the. Then a zinc electrode is placed in the zinc sulfate solution. A typical cell might consist of two pieces of metal, one zinc and the other. Copper And Zinc Electrochemical Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Copper And Zinc Electrochemical Cell Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The salt bridge prevents the. In a spontaneous reaction electrons leave the zinc, go through the wire and are. Copper And Zinc Electrochemical Cell.
From www.pinterest.com
Kognity Chemistry classroom, Electrochemistry, Chemistry lessons Copper And Zinc Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. Use cell notation to describe the galvanic cell where. Copper And Zinc Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Copper And Zinc Electrochemical Cell In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. A zinc sulfate solution is floated on top of the copper sulfate solution; For the metallic components (zinc, copper, copper. In the process of the reaction,. The salt bridge prevents the. One reactant gives up electrons (undergoes oxidation) and. Copper And Zinc Electrochemical Cell.
From tecnico.aspillagahornauer.cl
Electrochemical Cells, 41 OFF Copper And Zinc Electrochemical Cell A zinc sulfate solution is floated on top of the copper sulfate solution; For the metallic components (zinc, copper, copper. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc. Copper And Zinc Electrochemical Cell.
From www.alamy.com
Copper zinc cell Cut Out Stock Images & Pictures Alamy Copper And Zinc Electrochemical Cell For the metallic components (zinc, copper, copper. In the process of the reaction,. A zinc sulfate solution is floated on top of the copper sulfate solution; Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The salt bridge prevents the. One reactant gives up electrons. Copper And Zinc Electrochemical Cell.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Copper And Zinc Electrochemical Cell A zinc sulfate solution is floated on top of the copper sulfate solution; One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. The salt bridge prevents the. Use cell notation to describe the galvanic cell where. Copper And Zinc Electrochemical Cell.
From saylordotorg.github.io
Describing Electrochemical Cells Copper And Zinc Electrochemical Cell The salt bridge prevents the. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. Then a zinc electrode is placed in the zinc sulfate solution. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc. Copper And Zinc Electrochemical Cell.
From stock.adobe.com
Electrochemical cell diagram. Galvanic cell or voltaic cell. Zinc anode Copper And Zinc Electrochemical Cell A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The processes responsible for the current flow in an electrochemical cell depend on. Copper And Zinc Electrochemical Cell.
From www.slideserve.com
PPT Chapter 22 Electrochemistry PowerPoint Presentation, free Copper And Zinc Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The salt bridge prevents the. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. For the metallic components (zinc, copper, copper. A zinc sulfate solution is floated. Copper And Zinc Electrochemical Cell.
From www.alamy.com
Daniell element galvanic cell with zinc and copper Stock Photo Copper And Zinc Electrochemical Cell Then a zinc electrode is placed in the zinc sulfate solution. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. For the metallic components (zinc, copper, copper. In the process of the reaction,. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A typical cell. Copper And Zinc Electrochemical Cell.
From ar.inspiredpencil.com
Copper Electrolytic Cell Copper And Zinc Electrochemical Cell In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. In the process of the reaction,. For the metallic components (zinc, copper, copper. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The salt bridge prevents the. Use cell notation to. Copper And Zinc Electrochemical Cell.
From www.freepik.com
Premium Photo Electrochemical cell or Galvanic cell. The Daniell cell Copper And Zinc Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. A zinc sulfate solution is floated on top of. Copper And Zinc Electrochemical Cell.
From quizlet.com
Label the zinc/copper galvanic cell Diagram Quizlet Copper And Zinc Electrochemical Cell The salt bridge prevents the. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. In the process of the reaction,. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. For the metallic components. Copper And Zinc Electrochemical Cell.
From www.youtube.com
ZincCopper Electrochemical Cell Demonstration YouTube Copper And Zinc Electrochemical Cell The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. A zinc sulfate solution is floated on top of the copper sulfate solution; For the metallic components (zinc, copper, copper. A typical cell might consist of two. Copper And Zinc Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID1195562 Copper And Zinc Electrochemical Cell A zinc sulfate solution is floated on top of the copper sulfate solution; In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. The processes responsible for the current flow in an. Copper And Zinc Electrochemical Cell.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Copper And Zinc Electrochemical Cell A zinc sulfate solution is floated on top of the copper sulfate solution; The salt bridge prevents the. In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. One reactant gives up. Copper And Zinc Electrochemical Cell.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Copper And Zinc Electrochemical Cell For the metallic components (zinc, copper, copper. One reactant gives up electrons (undergoes oxidation) and another reactant gains electrons. In the process of the reaction,. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. A typical cell might consist of two pieces of metal, one. Copper And Zinc Electrochemical Cell.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Copper And Zinc Electrochemical Cell In a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. In the process of the reaction,. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A zinc sulfate. Copper And Zinc Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Copper And Zinc Electrochemical Cell A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulfates. For the metallic components (zinc, copper, copper. Then a zinc electrode is placed. Copper And Zinc Electrochemical Cell.
From www.scienceabc.com
(Photo Credit Nandalal Sarkar/Shutterstock) Copper And Zinc Electrochemical Cell In the process of the reaction,. A zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution. The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. The salt bridge prevents the. In a spontaneous reaction. Copper And Zinc Electrochemical Cell.