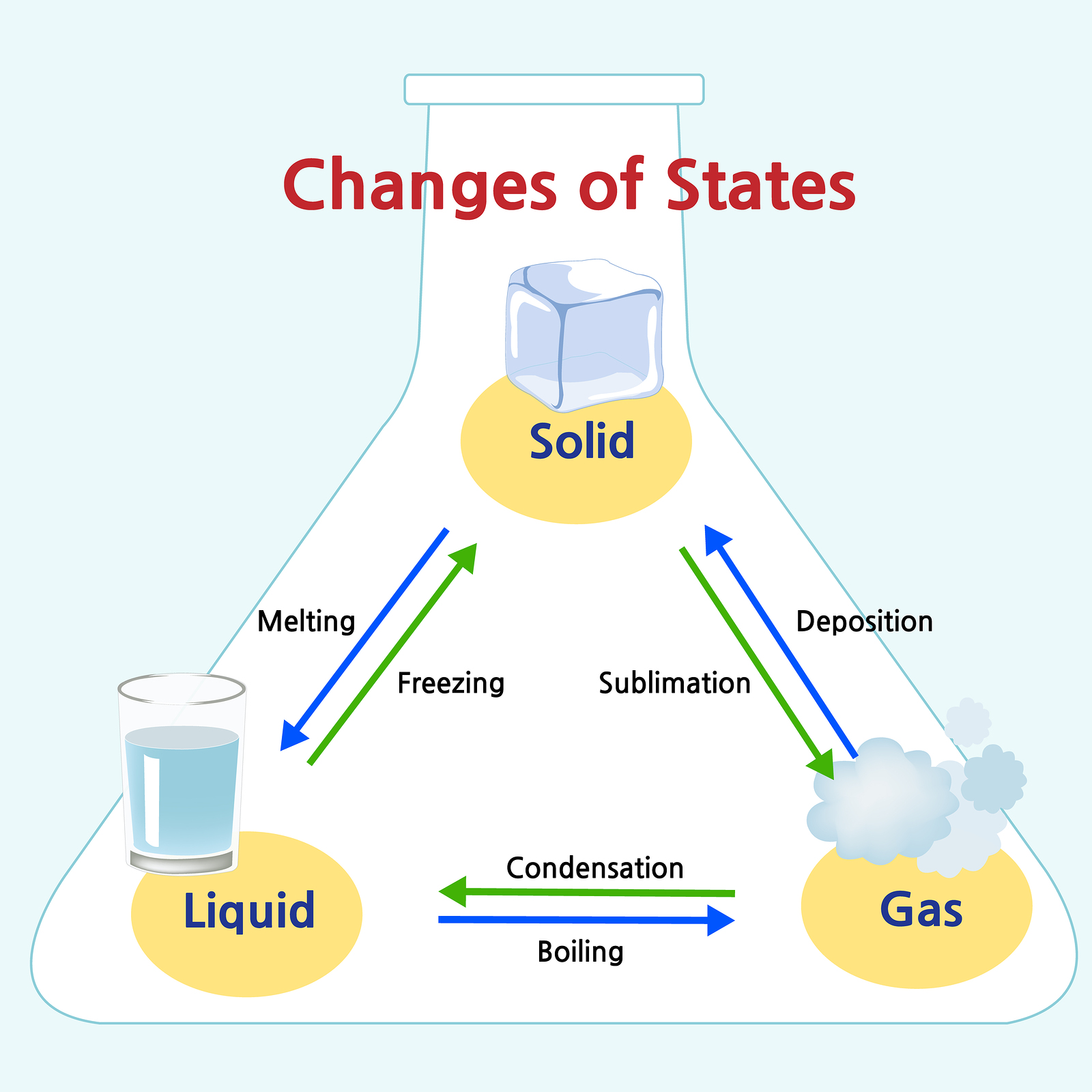
Solid Changes To Liquid . Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. Solids form by deposition from gases or freezing of liquids. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and. Both the solid and liquid states of a substance coexist in equilibrium during the change of state. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. The process of obtaining solid from the liquid is the opposite; Liquids can vaporize into gases or freeze into solids. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Melting, freezing, evaporating and condensing. Changes of state between solid and liquid. Every element and substance can transition from one phase to another at a. There are four main changes of state:
from www.theschoolrun.com
There are four main changes of state: Every element and substance can transition from one phase to another at a. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Melting, freezing, evaporating and condensing. Liquids can vaporize into gases or freeze into solids. Changes of state between solid and liquid. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Both the solid and liquid states of a substance coexist in equilibrium during the change of state. The process of obtaining solid from the liquid is the opposite; Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating.
What are states of matter? TheSchoolRun
Solid Changes To Liquid The process of obtaining solid from the liquid is the opposite; Solids form by deposition from gases or freezing of liquids. Both the solid and liquid states of a substance coexist in equilibrium during the change of state. The process of obtaining solid from the liquid is the opposite; Melting, freezing, evaporating and condensing. There are four main changes of state: Changes of state between solid and liquid. Every element and substance can transition from one phase to another at a. Liquids can vaporize into gases or freeze into solids. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Solid Changes To Liquid Liquids can vaporize into gases or freeze into solids. Changes of state between solid and liquid. The process of obtaining solid from the liquid is the opposite; There are four main changes of state: Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Solids. Solid Changes To Liquid.
From sites.google.com
Chemistry Jones J Solid Changes To Liquid Solids form by deposition from gases or freezing of liquids. Every element and substance can transition from one phase to another at a. Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. Both the solid and liquid states of a substance coexist in equilibrium during the change of state. The. Solid Changes To Liquid.
From www.radixtree.com
Physics Matter Online Education System Solid Changes To Liquid Melting, freezing, evaporating and condensing. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. Liquids can vaporize into gases or freeze into solids. Every element. Solid Changes To Liquid.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Science Blog Solid Changes To Liquid Melting, freezing, evaporating and condensing. Every element and substance can transition from one phase to another at a. Both the solid and liquid states of a substance coexist in equilibrium during the change of state. The process of obtaining solid from the liquid is the opposite; Phase transition is when a substance changes from a solid, liquid, or gas state. Solid Changes To Liquid.
From www.teachoo.com
Interconversion of States of Matter with Flow Chart Teachoo Solid Changes To Liquid Liquids can vaporize into gases or freeze into solids. Every element and substance can transition from one phase to another at a. Changes of state between solid and liquid. The process of obtaining solid from the liquid is the opposite; Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid. Solid Changes To Liquid.
From www.youtube.com
Science 3 Quarter 1 Module 2 Lesson 1 Changes in Materials from Solid to Liquid YouTube Solid Changes To Liquid Solids form by deposition from gases or freezing of liquids. There are four main changes of state: Melting, freezing, evaporating and condensing. Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the. Solid Changes To Liquid.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid Changes To Liquid Changes of state between solid and liquid. Melting, freezing, evaporating and condensing. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Remember that particles in a. Solid Changes To Liquid.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The F... Matter science Solid Changes To Liquid There are four main changes of state: Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a. Changes of state between solid and liquid. Both the solid and liquid states of a substance coexist in equilibrium during the change. Solid Changes To Liquid.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Solid Changes To Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and. Every element and substance can transition from one phase to another at a. Remember that particles in a solid are fixed in position and although they can't move around,. Solid Changes To Liquid.
From www.vecteezy.com
Three different States of matter solid, liquid and gasuas state. Inter change of state of matter Solid Changes To Liquid Every element and substance can transition from one phase to another at a. Both the solid and liquid states of a substance coexist in equilibrium during the change of state. The process of obtaining solid from the liquid is the opposite; Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the. Solid Changes To Liquid.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid Changes To Liquid Both the solid and liquid states of a substance coexist in equilibrium during the change of state. Changes of state between solid and liquid. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Solids form by deposition from gases or freezing of liquids. Remember that particles in a solid are fixed. Solid Changes To Liquid.
From worksheetlistutu.z13.web.core.windows.net
Liquids And Solids Worksheet Solid Changes To Liquid Liquids can vaporize into gases or freeze into solids. The process of obtaining solid from the liquid is the opposite; There are four main changes of state: We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and. Solids form. Solid Changes To Liquid.
From www.snexplores.org
Explainer What are the different states of matter? Solid Changes To Liquid Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. Both the solid and liquid states of a substance coexist in equilibrium during the change of state. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by. Solid Changes To Liquid.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid Changes To Liquid Solids form by deposition from gases or freezing of liquids. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. There are four main changes of. Solid Changes To Liquid.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 Solid Changes To Liquid Solids form by deposition from gases or freezing of liquids. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Melting, freezing, evaporating and condensing. There are four main changes of state: The process of obtaining solid from the liquid is the opposite; Changes of. Solid Changes To Liquid.
From www.britannica.com
phase Definition & Facts Britannica Solid Changes To Liquid Every element and substance can transition from one phase to another at a. The process of obtaining solid from the liquid is the opposite; Melting, freezing, evaporating and condensing. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and.. Solid Changes To Liquid.
From www.vecteezy.com
Changing the state of matter from solid, liquid and gas due to temperature. Vector Illustration Solid Changes To Liquid Both the solid and liquid states of a substance coexist in equilibrium during the change of state. There are four main changes of state: The process of obtaining solid from the liquid is the opposite; Solids form by deposition from gases or freezing of liquids. Melting, freezing, evaporating and condensing. Remember that particles in a solid are fixed in position. Solid Changes To Liquid.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma Solid Changes To Liquid Solids form by deposition from gases or freezing of liquids. Melting, freezing, evaporating and condensing. There are four main changes of state: Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid. Solid Changes To Liquid.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid Changes To Liquid Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. There are four main changes of state: Solids form by deposition from gases or freezing of liquids. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing.. Solid Changes To Liquid.
From www.ase.org.uk
Solids, liquids and gases Solid Changes To Liquid The process of obtaining solid from the liquid is the opposite; Both the solid and liquid states of a substance coexist in equilibrium during the change of state. There are four main changes of state: Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing.. Solid Changes To Liquid.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Solid Changes To Liquid Changes of state between solid and liquid. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. Both the solid and liquid states of a substance. Solid Changes To Liquid.
From www.theschoolrun.com
What are states of matter? TheSchoolRun Solid Changes To Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and. Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. Phase transition is when a substance changes from a solid,. Solid Changes To Liquid.
From emedia.rmit.edu.au
States of matter Learning Lab Solid Changes To Liquid Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and. Solids form by deposition from gases or freezing of liquids. Go. Solid Changes To Liquid.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid Changes To Liquid Both the solid and liquid states of a substance coexist in equilibrium during the change of state. Solids form by deposition from gases or freezing of liquids. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Changes of state between solid and liquid. We. Solid Changes To Liquid.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Illustration Solid Changes To Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and. Every element and substance can transition from one phase to another at a. Go in the reverse direction and you can change a gas into a liquid by condensation,. Solid Changes To Liquid.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Solid Changes To Liquid Liquids can vaporize into gases or freeze into solids. Changes of state between solid and liquid. Solids form by deposition from gases or freezing of liquids. Both the solid and liquid states of a substance coexist in equilibrium during the change of state. There are four main changes of state: Phase transition is when a substance changes from a solid,. Solid Changes To Liquid.
From sciencenotes.org
States of Matter Solid Changes To Liquid Melting, freezing, evaporating and condensing. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The process of obtaining solid from the liquid is the opposite; There are four main changes of state: Both the solid and liquid states of a substance coexist in equilibrium during the change of state. Go in. Solid Changes To Liquid.
From kids.britannica.com
solid Students Britannica Kids Homework Help Solid Changes To Liquid Changes of state between solid and liquid. Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. There are four main changes of state: Both the solid and liquid states of a substance coexist in equilibrium during the change of state. Go in the reverse direction and you can change a. Solid Changes To Liquid.
From saylordotorg.github.io
Solids and Liquids Solid Changes To Liquid Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Remember that particles in a solid are fixed in position and although they can't move around, they are vibrating. Both the solid and liquid states of a substance coexist in equilibrium during the change of. Solid Changes To Liquid.
From stock.adobe.com
Vector diagram with changing states of matter, three states of matter with different molecular Solid Changes To Liquid We take advantage of changes between the gas, liquid, and solid states to cool a drink with ice cubes (solid to liquid), cool our bodies by perspiration (liquid to gas), and. Melting, freezing, evaporating and condensing. Solids form by deposition from gases or freezing of liquids. Liquids can vaporize into gases or freeze into solids. Changes of state between solid. Solid Changes To Liquid.
From www.alamy.com
Water States of matter Phase. Change of State for Water Diagram. Changing the state of matter Solid Changes To Liquid Melting, freezing, evaporating and condensing. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Solids form by deposition from gases or freezing of liquids. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. We take. Solid Changes To Liquid.
From www.thoughtco.com
List of Phase Changes Between States of Matter Solid Changes To Liquid There are four main changes of state: The process of obtaining solid from the liquid is the opposite; Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Both the solid and liquid states of a substance coexist in equilibrium during the change of state.. Solid Changes To Liquid.
From wirelistlatinised.z21.web.core.windows.net
Solid Liquid Gas Diagram Solid Changes To Liquid Changes of state between solid and liquid. There are four main changes of state: Melting, freezing, evaporating and condensing. Liquids can vaporize into gases or freeze into solids. Go in the reverse direction and you can change a gas into a liquid by condensation, then turn the liquid into a solid by freezing. Every element and substance can transition from. Solid Changes To Liquid.
From manuallistcantabank.z21.web.core.windows.net
Solid Liquid And Gas Diagram Solid Changes To Liquid Solids form by deposition from gases or freezing of liquids. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Both the solid and liquid states of a substance coexist in equilibrium during the change of state. Every element and substance can transition from one phase to another at a. Melting, freezing,. Solid Changes To Liquid.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Solid Changes To Liquid Changes of state between solid and liquid. Solids form by deposition from gases or freezing of liquids. The process of obtaining solid from the liquid is the opposite; Melting, freezing, evaporating and condensing. There are four main changes of state: Liquids can vaporize into gases or freeze into solids. Remember that particles in a solid are fixed in position and. Solid Changes To Liquid.