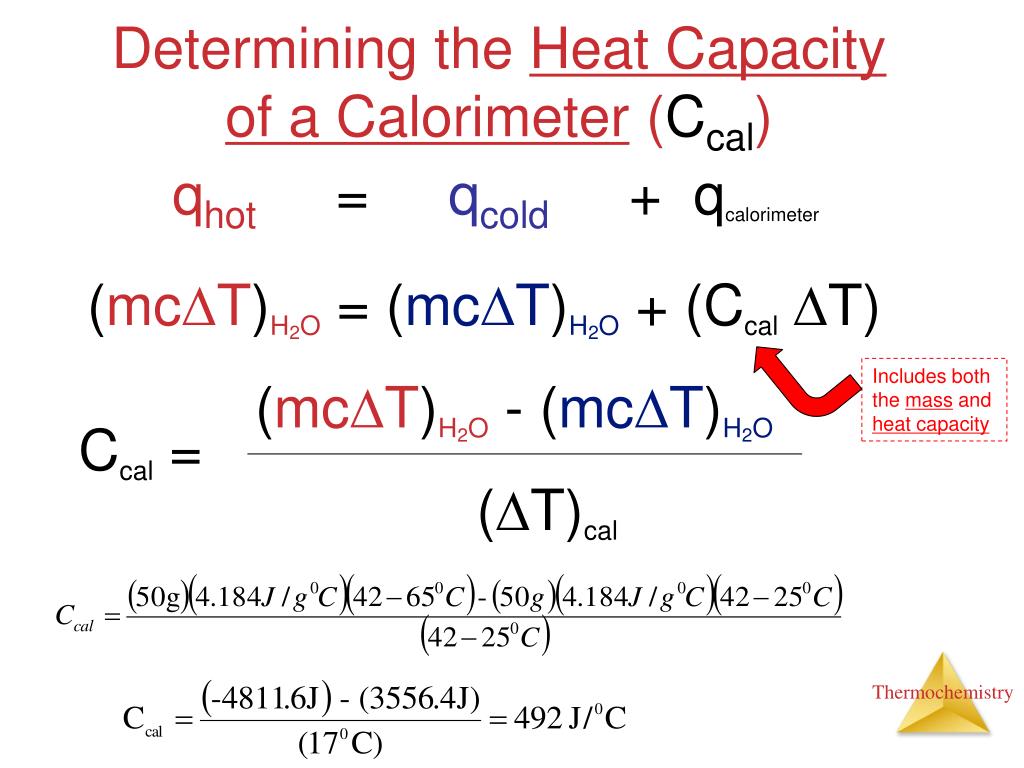
Calorimetry Change Formula . To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). Δt denotes the temperature change. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; Calculate the molar heat of enthalpy for a. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and.
from www.slideserve.com
Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; Calculate the molar heat of enthalpy for a. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. To do so, the heat is exchanged with a calibrated object (calorimeter). Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance.
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download
Calorimetry Change Formula To do so, the heat is exchanged with a calibrated object (calorimeter). Calculate the molar heat of enthalpy for a. Calorimetry is used to measure amounts of heat transferred to or from a substance. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. Δt denotes the temperature change. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; To do so, the heat is exchanged with a calibrated object. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimetry Change Formula Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. The formula q = mcδt is used to calculate the heat energy transferred in. Calorimetry Change Formula.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Change Formula The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to or from a substance. Δt denotes the temperature change. To do so, the heat is exchanged with a calibrated. Calorimetry Change Formula.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Change Formula Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure. Calorimetry Change Formula.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Change Formula Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the molar heat of enthalpy for a. Calorimetry is used to measure amounts of heat transferred to or from a substance. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. To do so, the heat is exchanged with. Calorimetry Change Formula.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Change Formula Δt denotes the temperature change. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,.. Calorimetry Change Formula.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube Calorimetry Change Formula To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; Calculate the molar heat of enthalpy for a. Calorimetry is used to measure amounts of heat transferred to or from a. Calorimetry Change Formula.
From www.youtube.com
How to find calorimeter constant YouTube Calorimetry Change Formula Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and. Calorimetry Change Formula.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry Change Formula Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calorimetry is used to measure amounts of heat transferred to or from a substance. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. To do so, the heat is. Calorimetry Change Formula.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Change Formula To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry measures enthalpy changes during chemical processes, where the magnitude of. Calorimetry Change Formula.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Change Formula To do so, the heat is exchanged with a calibrated object. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Δt denotes the temperature change. Calorimetry measures enthalpy changes. Calorimetry Change Formula.
From www.youtube.com
Delta H and Calorimetry YouTube Calorimetry Change Formula To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. The formula q = mcδt. Calorimetry Change Formula.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Change Formula Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. Calorimetry is used to measure amounts of heat transferred to or from a substance. To. Calorimetry Change Formula.
From www.wizeprep.com
Calorimetry Wize University Chemistry Textbook Wizeprep Calorimetry Change Formula Calorimetry is used to measure amounts of heat transferred to or from a substance. Δt denotes the temperature change. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. Calculate heat, temperature change, and specific heat after thermal equilibrium is. Calorimetry Change Formula.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Calorimetry Change Formula Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry measures enthalpy changes during chemical processes, where. Calorimetry Change Formula.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID197266 Calorimetry Change Formula To do so, the heat is exchanged with a calibrated object (calorimeter). Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Δt denotes the. Calorimetry Change Formula.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Change Formula Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calculate the molar heat of enthalpy for a. Δt denotes the temperature change.. Calorimetry Change Formula.
From slideplayer.com
Calorimetry. ppt download Calorimetry Change Formula To do so, the heat is exchanged. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the. Calorimetry Change Formula.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Change Formula To do so, the heat is exchanged with a calibrated object (calorimeter). To do so, the heat is exchanged. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy. Calorimetry Change Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimetry Change Formula Δt denotes the temperature change. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. Another common unit of energy often used for heat is the. Calorimetry Change Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 Calorimetry Change Formula Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. To do so, the heat is exchanged with a calibrated object. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. Calorimetry is used. Calorimetry Change Formula.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 Calorimetry Change Formula Calorimetry is used to measure amounts of heat transferred to or from a substance. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. To do so, the heat is exchanged with a calibrated object (calorimeter). Calculate the molar heat. Calorimetry Change Formula.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Change Formula Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Δt denotes the temperature change. The formula q = mcδt is used to calculate the heat energy transferred in. Calorimetry Change Formula.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Change Formula Calculate the molar heat of enthalpy for a. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat. Calorimetry Change Formula.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Change Formula Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Another common unit of energy often used for heat. Calorimetry Change Formula.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimetry Change Formula The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Δt denotes the temperature change. Calorimetry is used to measure amounts of heat transferred to or from a substance.. Calorimetry Change Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Change Formula Calculate the molar heat of enthalpy for a. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. To do so, the heat is exchanged. Calorimetry Change Formula.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Change Formula Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. To do so, the heat is exchanged with a calibrated object. Calorimetry measures enthalpy changes during. Calorimetry Change Formula.
From www.numerade.com
SOLVEDThe defining equation for calorimetry is Calorimetry Change Formula Calculate the molar heat of enthalpy for a. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. Δt denotes the temperature change. To do so, the heat is exchanged with a calibrated object (calorimeter). The formula q = mcδt is used to calculate the heat energy transferred in. Calorimetry Change Formula.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Calorimetry Change Formula Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; To do so, the heat is exchanged. The formula q = mcδt is used. Calorimetry Change Formula.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimetry Change Formula Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. Calorimetry is used to. Calorimetry Change Formula.
From www.youtube.com
Coffee Cup Calorimeter Calculate Enthalpy Change, Constant Pressure Calorimetry Change Formula Δt denotes the temperature change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature. Calorimetry Change Formula.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Change Formula Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. The formula q = mcδt is used to calculate the heat energy. Calorimetry Change Formula.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimetry Change Formula Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. The formula q = mcδt is used to calculate the heat energy transferred in a calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calorimetry Change Formula.
From www.youtube.com
Calorimetry Part 2 calculations YouTube Calorimetry Change Formula Calculate the molar heat of enthalpy for a. Δt denotes the temperature change. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the. Calorimetry Change Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5577734 Calorimetry Change Formula Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \ (1.00^oc\)—specifically,. Δt denotes the temperature change. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter; To do so, the heat is exchanged. Calorimetry Change Formula.