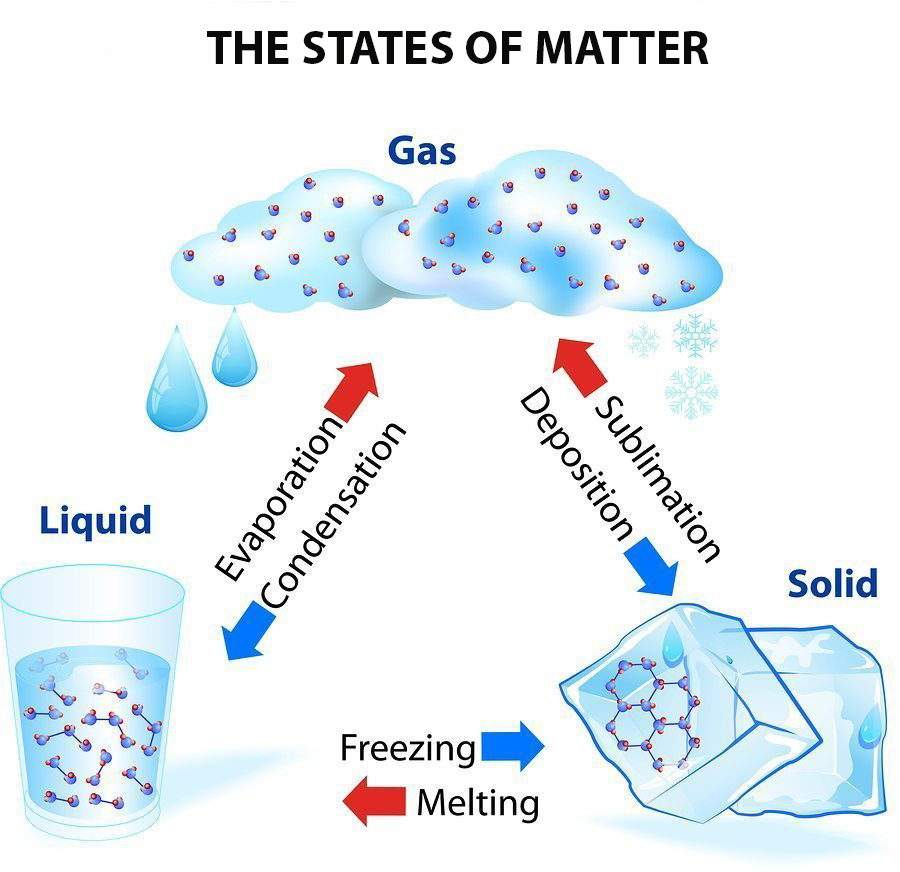
In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following . In a solution of a solid in a liquid, the solid is called which of the following? Solvents can also be gases, liquids or solids. For example, a solute can be a gas, a liquid or a solid. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. An aqueous solution is water that contains one or more. The following figures show the. Describe the effects of temperature and pressure on solubility; In this chapter, we will focus on solution where the solvent is water. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution.
from itinerantmission.blogspot.com
Describe the effects of temperature and pressure on solubility; Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. An aqueous solution is water that contains one or more. The following figures show the. In this chapter, we will focus on solution where the solvent is water. In a solution of a solid in a liquid, the solid is called which of the following? For example, a solute can be a gas, a liquid or a solid. Solvents can also be gases, liquids or solids.
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas
In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following For example, a solute can be a gas, a liquid or a solid. Solvents can also be gases, liquids or solids. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. In a solution of a solid in a liquid, the solid is called which of the following? Describe the effects of temperature and pressure on solubility; When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. The following figures show the. In this chapter, we will focus on solution where the solvent is water. For example, a solute can be a gas, a liquid or a solid. An aqueous solution is water that contains one or more.
From devinitionva.blogspot.com
Definition Of Solute Solvent And Solution DEFINITIONVA In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following In this chapter, we will focus on solution where the solvent is water. Solvents can also be gases, liquids or solids. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. The following figures show the. Describe the effects of temperature and pressure on solubility; In a. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following For example, a solute can be a gas, a liquid or a solid. An aqueous solution is water that contains one or more. In this chapter, we will focus on solution where the solvent is water. In a solution of a solid in a liquid, the solid is called which of the following? Describe the effects of temperature and pressure. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From owlcation.com
All About Matter An Introduction to the Basics Owlcation In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following For example, a solute can be a gas, a liquid or a solid. Solvents can also be gases, liquids or solids. An aqueous solution is water that contains one or more. In this chapter, we will focus on solution where the solvent is water. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.askiitians.com
Classification of Solids Study Material for IIT JEE askIITians In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following The following figures show the. In this chapter, we will focus on solution where the solvent is water. Solvents can also be gases, liquids or solids. An aqueous solution is water that contains one or more. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. Describe the effects. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.youtube.com
Factors Affecting Solubility of Solid in Liquid Solution and In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Describe the effects of temperature and pressure on solubility; Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. The following figures show the. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. An aqueous. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From mungfali.com
Solid Liquid Gas Anchor Chart In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. An aqueous solution is water that contains one or more. In this chapter, we will focus on solution where the solvent is water. Solvents can also be gases, liquids or solids. Therefore, if only two chemicals are present in. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.youtube.com
Separation of solidliquid mixtures YouTube In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. The following figures show the. An aqueous solution is water that contains one or more. In this chapter, we will focus on solution where the solvent is water. For example, a solute can be a gas, a liquid or. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.youtube.com
Changes in Materials when Mixed with other Materials (Part 3 Solid to In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following In a solution of a solid in a liquid, the solid is called which of the following? Solvents can also be gases, liquids or solids. Describe the effects of temperature and pressure on solubility; An aqueous solution is water that contains one or more. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.ase.org.uk
Solids, liquids and gases In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following The following figures show the. Solvents can also be gases, liquids or solids. In a solution of a solid in a liquid, the solid is called which of the following? An aqueous solution is water that contains one or more. In this chapter, we will focus on solution where the solvent is water. For example, a solute can be a. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From wirelistlatinised.z21.web.core.windows.net
Solid Liquid Gas Diagram In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Solvents can also be gases, liquids or solids. In a solution of a solid in a liquid, the solid is called which of the following? Describe the effects of temperature and pressure on solubility; When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. An aqueous solution is water. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.youtube.com
Solid in liquid solution YouTube In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following An aqueous solution is water that contains one or more. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. In this chapter, we will focus. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.snexplores.org
Explainer What are the different states of matter? In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following In this chapter, we will focus on solution where the solvent is water. In a solution of a solid in a liquid, the solid is called which of the following? Solvents can also be gases, liquids or solids. For example, a solute can be a gas, a liquid or a solid. The following figures show the. Describe the effects of. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From examples.yourdictionary.com
Examples of Homogeneous Mixtures Solid, Liquid and Gas YourDictionary In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following For example, a solute can be a gas, a liquid or a solid. An aqueous solution is water that contains one or more. In a solution of a solid in a liquid, the solid is called which of the following? In this chapter, we will focus on solution where the solvent is water. Describe the effects of temperature and pressure. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.dreamstime.com
Solution. Solid In Liquid Stock Vector Image 67386593 In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Solvents can also be gases, liquids or solids. In this chapter, we will focus on solution where the solvent is water. The following figures show the. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. In a solution of a solid in a liquid, the solid. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.britannica.com
phase Definition & Facts Britannica In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following An aqueous solution is water that contains one or more. Solvents can also be gases, liquids or solids. Describe the effects of temperature and pressure on solubility; The following figures show the. In a solution of a solid in a liquid, the solid is called which of the following? For example, a solute can be a gas, a liquid or. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.solnpharma.com
Difference Between Solid and Liquid Mixing In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following For example, a solute can be a gas, a liquid or a solid. The following figures show the. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. Describe the effects of temperature and pressure on solubility; An aqueous solution is water that contains one or more. In this. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From kids.britannica.com
solid Students Britannica Kids Homework Help In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Solvents can also be gases, liquids or solids. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. The following figures show the. In this chapter, we will focus on solution where the solvent is water. Describe the effects of temperature and pressure on solubility; For example, a solute. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. Describe the effects of temperature and pressure on solubility; When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. An aqueous solution is water that contains. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From byjus.com
31. If we form a solution by mixing a solid and a liquid, and the In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following For example, a solute can be a gas, a liquid or a solid. Solvents can also be gases, liquids or solids. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. An aqueous solution is water that contains one or more. Describe the effects of temperature and. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.studypool.com
SOLUTION Solid liquid and gas Studypool In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Solvents can also be gases, liquids or solids. An aqueous solution is water that contains one or more. In a solution of a solid in a liquid, the solid is called which of the following? For example, a solute can be a gas, a liquid or a solid. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.youtube.com
Separation of solid liquid mixtureCrystallisation YouTube In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. An aqueous solution is water that contains one or more. In a solution of a solid. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.youtube.com
SOLID LIQUID MIXTURE YouTube In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following For example, a solute can be a gas, a liquid or a solid. Solvents can also be gases, liquids or solids. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. In a solution of a solid in a liquid, the solid is called which of the following? An. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.dreamstime.com
Solution Solid in liquid stock vector. Illustration of mixtures In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following An aqueous solution is water that contains one or more. The following figures show the. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. For example, a solute can be a gas, a liquid or a solid. Describe the effects of temperature and pressure on solubility;. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.bartleby.com
Answered The changing of a liquid to a solid is… bartleby In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following The following figures show the. Solvents can also be gases, liquids or solids. In this chapter, we will focus on solution where the solvent is water. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. For example, a solute can be a gas, a liquid or a solid.. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.chegg.com
Solved The following animations depict the movement of solid In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Solvents can also be gases, liquids or solids. Describe the effects of temperature and pressure on solubility; In this chapter, we will focus on solution where the solvent is water. For example, a solute can be a gas, a liquid or a solid. The following figures show the. An aqueous solution is water that contains one or more. In a. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From mavink.com
Parts Of Matter In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. Solvents can also be gases, liquids or solids. For example, a solute can be a gas, a liquid or a solid. In a solution of a solid in a liquid, the solid is called which of the following? An. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.youtube.com
DIY Solid Liquid Mixture YouTube In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Solvents can also be gases, liquids or solids. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. For example, a solute can be a gas, a liquid or a solid. Describe the effects of temperature and pressure on solubility; In a solution of a solid in. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.etsy.com
Science, States of Matter, Solid, Liquid, Gas, Elementary, Anchor Chart In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. In this chapter, we will focus on solution where the solvent is water. For example, a. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.pinterest.co.uk
Liquid Definition Examples of Liquids Solid liquid gas, States of In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Describe the effects of temperature and pressure on solubility; In this chapter, we will focus on solution where the solvent is water. For example, a solute can be a gas, a liquid or a solid. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. Therefore, if only two. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.youtube.com
SEPARATION OF SOLID LIQUID MIXTURECLASS 7 YouTube In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following An aqueous solution is water that contains one or more. In this chapter, we will focus on solution where the solvent is water. For example, a solute can be a gas, a liquid or a solid. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. In. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From courses.lumenlearning.com
The Dissolution Process Chemistry In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. Therefore, if only two chemicals are present in a solution, one substance serves as the solvent and the other acts as a solute. Describe the effects of temperature and pressure on solubility; An aqueous solution is water that contains. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Solvents can also be gases, liquids or solids. For example, a solute can be a gas, a liquid or a solid. In this chapter, we will focus on solution where the solvent is water. In a solution of a solid in a liquid, the solid is called which of the following? An aqueous solution is water that contains one or. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From www.studypool.com
SOLUTION Examples of solid liquid gas Studypool In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following Describe the effects of temperature and pressure on solubility; In this chapter, we will focus on solution where the solvent is water. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must be overcome to form a solution. Solvents can also be gases, liquids or solids. Therefore, if only two chemicals are present in a. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following In a solution of a solid in a liquid, the solid is called which of the following? The following figures show the. An aqueous solution is water that contains one or more. For example, a solute can be a gas, a liquid or a solid. Solvents can also be gases, liquids or solids. Therefore, if only two chemicals are present. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.
From brainly.in
Give two examples for each of the following • solid + solid mixture In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following The following figures show the. In a solution of a solid in a liquid, the solid is called which of the following? For example, a solute can be a gas, a liquid or a solid. An aqueous solution is water that contains one or more. When the solute is an ionic solid, \(δh_2\) corresponds to the lattice energy that must. In A Solution Of A Solid In A Liquid The Solid Is Called Which Of The Following.