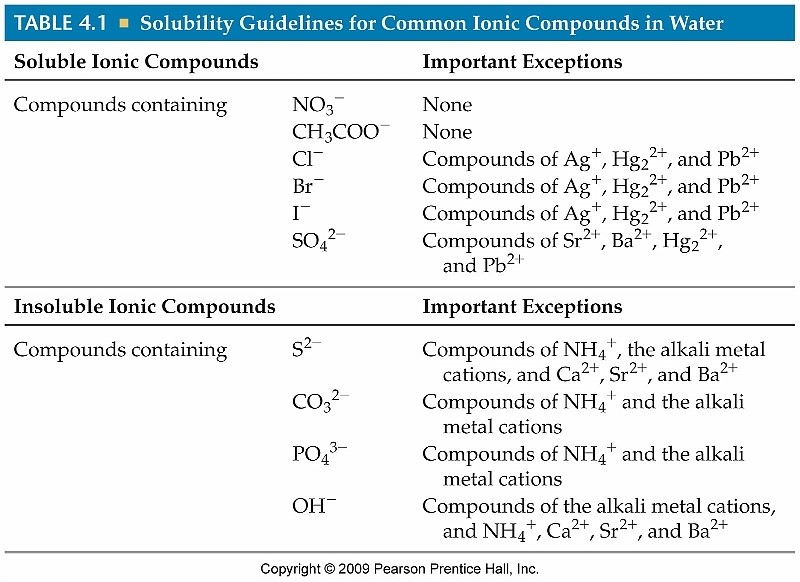
Which Covalent Compounds Are Soluble In Water . When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. This means that substances with the same type of polarity will be soluble in one another. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. All nitrates are soluble in water so zn (no 3) 2 is soluble. Water is a polar solvent, but covalent compounds are usually nonpolar. Our article on solubility explains how ionic compounds dissolve in water. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. The following sections discuss both soluble and insoluble compounds in water. There are several major types of compounds that are soluble in water.
from chemcollective.org
When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. Our article on solubility explains how ionic compounds dissolve in water. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. Water is a polar solvent, but covalent compounds are usually nonpolar. There are several major types of compounds that are soluble in water. This means that substances with the same type of polarity will be soluble in one another. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. The following sections discuss both soluble and insoluble compounds in water.
CHEM 1315 Lab 10 Conservation of Mass
Which Covalent Compounds Are Soluble In Water When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. This means that substances with the same type of polarity will be soluble in one another. The following sections discuss both soluble and insoluble compounds in water. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. There are several major types of compounds that are soluble in water. All nitrates are soluble in water so zn (no 3) 2 is soluble. Our article on solubility explains how ionic compounds dissolve in water. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. Water is a polar solvent, but covalent compounds are usually nonpolar.
From www.youtube.com
Organic Chemistry. Solubility in Water YouTube Which Covalent Compounds Are Soluble In Water Our article on solubility explains how ionic compounds dissolve in water. This means that substances with the same type of polarity will be soluble in one another. All nitrates are soluble in water so zn (no 3) 2 is soluble. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate.. Which Covalent Compounds Are Soluble In Water.
From www.thoughtco.com
Examples of Covalent Bonds and Compounds Which Covalent Compounds Are Soluble In Water Our article on solubility explains how ionic compounds dissolve in water. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. All nitrates are soluble in water so zn (no 3) 2 is soluble. All. Which Covalent Compounds Are Soluble In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Which Covalent Compounds Are Soluble In Water The following sections discuss both soluble and insoluble compounds in water. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. There are several major types of compounds that are soluble in water. Our article on solubility explains how ionic compounds dissolve in water. When covalent compounds dissolve in water they break apart. Which Covalent Compounds Are Soluble In Water.
From www.youtube.com
Predicting solubility of ionic compounds in water YouTube Which Covalent Compounds Are Soluble In Water Polar species are soluble in water, while nonpolar species are soluble in oils and fats. There are several major types of compounds that are soluble in water. Water is a polar solvent, but covalent compounds are usually nonpolar. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. When solute mixes with. Which Covalent Compounds Are Soluble In Water.
From www.numerade.com
SOLVEDWhat kinds of covalent compounds are soluble in water? Which Covalent Compounds Are Soluble In Water All nitrates are soluble in water so zn (no 3) 2 is soluble. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. The following sections discuss both soluble and insoluble compounds in water. There. Which Covalent Compounds Are Soluble In Water.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Which Covalent Compounds Are Soluble In Water This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. This means that substances with the same type of polarity will be soluble in one another. There are several major types of compounds that are soluble in. Which Covalent Compounds Are Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Which Covalent Compounds Are Soluble In Water This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. Our article on solubility explains how ionic compounds dissolve in water. When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. This means that substances with the same type of polarity will be soluble in one another.. Which Covalent Compounds Are Soluble In Water.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Which Covalent Compounds Are Soluble In Water This means that substances with the same type of polarity will be soluble in one another. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. Water is a polar solvent, but covalent compounds are usually nonpolar. All nitrates are soluble in water so zn (no 3) 2 is soluble.. Which Covalent Compounds Are Soluble In Water.
From smallbusinessron.web.fc2.com
do covalent compounds dissolve in water Which Covalent Compounds Are Soluble In Water Our article on solubility explains how ionic compounds dissolve in water. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. The. Which Covalent Compounds Are Soluble In Water.
From surfguppy.com
What is Polar Covalent Bond Which Covalent Compounds Are Soluble In Water When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. This means covalent compounds typically don't dissolve in. Which Covalent Compounds Are Soluble In Water.
From www.slideserve.com
PPT Test Chapter 3 campbell’s 7 th ed Friday, August 31, 2012 Which Covalent Compounds Are Soluble In Water This means that substances with the same type of polarity will be soluble in one another. All nitrates are soluble in water so zn (no 3) 2 is soluble. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. When covalent compounds dissolve in water they break apart into molecules,. Which Covalent Compounds Are Soluble In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson Which Covalent Compounds Are Soluble In Water This means that substances with the same type of polarity will be soluble in one another. Water is a polar solvent, but covalent compounds are usually nonpolar. When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. All nitrates are soluble in water so zn (no 3) 2 is soluble. There are several major types. Which Covalent Compounds Are Soluble In Water.
From socratic.org
What determines whether a solid is soluble in water? Socratic Which Covalent Compounds Are Soluble In Water This means that substances with the same type of polarity will be soluble in one another. Our article on solubility explains how ionic compounds dissolve in water. The following sections discuss both soluble and insoluble compounds in water. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. This means. Which Covalent Compounds Are Soluble In Water.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download Which Covalent Compounds Are Soluble In Water Water is a polar solvent, but covalent compounds are usually nonpolar. All nitrates are soluble in water so zn (no 3) 2 is soluble. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain. Which Covalent Compounds Are Soluble In Water.
From www.youtube.com
which molecule is most soluble in water? YouTube Which Covalent Compounds Are Soluble In Water Our article on solubility explains how ionic compounds dissolve in water. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. There are several major types of compounds that are soluble in water. The following sections discuss both soluble and insoluble compounds in water. This means that substances with the. Which Covalent Compounds Are Soluble In Water.
From www.numerade.com
SOLVED Predict which of these covalent compounds is soluble in water Which Covalent Compounds Are Soluble In Water All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. There are several major types of compounds that are soluble in water. All nitrates are soluble in water so zn (no 3) 2 is soluble. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. This means covalent. Which Covalent Compounds Are Soluble In Water.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps Which Covalent Compounds Are Soluble In Water This means that substances with the same type of polarity will be soluble in one another. There are several major types of compounds that are soluble in water. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's. Which Covalent Compounds Are Soluble In Water.
From www.slideserve.com
PPT Water Solutions & Colligative Properties PowerPoint Presentation Which Covalent Compounds Are Soluble In Water All nitrates are soluble in water so zn (no 3) 2 is soluble. Our article on solubility explains how ionic compounds dissolve in water. This means that substances with the same type of polarity will be soluble in one another. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. All bromides are soluble in. Which Covalent Compounds Are Soluble In Water.
From www.alamy.com
Diagram to illustrate covalent bonding in water with a fully labelled Which Covalent Compounds Are Soluble In Water Polar species are soluble in water, while nonpolar species are soluble in oils and fats. There are several major types of compounds that are soluble in water. The following sections discuss both soluble and insoluble compounds in water. This means that substances with the same type of polarity will be soluble in one another. All bromides are soluble in water,. Which Covalent Compounds Are Soluble In Water.
From oerpub.github.io
This figure shows the structure of a water molecule. The top panel Which Covalent Compounds Are Soluble In Water When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. This means that. Which Covalent Compounds Are Soluble In Water.
From smallbusinessron.web.fc2.com
do covalent compounds dissolve in water Which Covalent Compounds Are Soluble In Water Our article on solubility explains how ionic compounds dissolve in water. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. There are several major types of compounds that are soluble in water. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a. Which Covalent Compounds Are Soluble In Water.
From chem.libretexts.org
4.1 General Properties of Aqueous Solutions Chemistry LibreTexts Which Covalent Compounds Are Soluble In Water For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. There are several major types of compounds that are soluble in water. All nitrates are soluble in water so zn (no 3) 2 is soluble.. Which Covalent Compounds Are Soluble In Water.
From www.slideserve.com
PPT Ionic and Covalent bonding PowerPoint Presentation, free download Which Covalent Compounds Are Soluble In Water When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. Our article on solubility explains how ionic compounds dissolve in water. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. There are several major types of compounds that are soluble in. Which Covalent Compounds Are Soluble In Water.
From chemcollective.org
CHEM 1315 Lab 10 Conservation of Mass Which Covalent Compounds Are Soluble In Water There are several major types of compounds that are soluble in water. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. When solute mixes with solvent, it can dissolve and form a solution or. Which Covalent Compounds Are Soluble In Water.
From bio.libretexts.org
2.1 Covalent Bonds Biology LibreTexts Which Covalent Compounds Are Soluble In Water The following sections discuss both soluble and insoluble compounds in water. All nitrates are soluble in water so zn (no 3) 2 is soluble. This means that substances with the same type of polarity will be soluble in one another. Water is a polar solvent, but covalent compounds are usually nonpolar. Our article on solubility explains how ionic compounds dissolve. Which Covalent Compounds Are Soluble In Water.
From www.chegg.com
Solved 10. In which set are both compounds soluble in water? Which Covalent Compounds Are Soluble In Water Water is a polar solvent, but covalent compounds are usually nonpolar. All nitrates are soluble in water so zn (no 3) 2 is soluble. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. Our article on solubility explains how ionic compounds dissolve in water. When solute mixes with solvent, it can dissolve. Which Covalent Compounds Are Soluble In Water.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Which Covalent Compounds Are Soluble In Water This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. Our article on solubility explains how ionic compounds dissolve in water. All nitrates are soluble in water so zn (no 3) 2 is soluble. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as. Which Covalent Compounds Are Soluble In Water.
From thechemistrynotes.com
Covalent Compounds Definition, Examples, Properties Which Covalent Compounds Are Soluble In Water Our article on solubility explains how ionic compounds dissolve in water. All nitrates are soluble in water so zn (no 3) 2 is soluble. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a. Which Covalent Compounds Are Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Which Covalent Compounds Are Soluble In Water When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. There are several major types of compounds that are soluble in water. This. Which Covalent Compounds Are Soluble In Water.
From www.slideserve.com
PPT Bonding What exactly is a bond? Depends…Ionic or Covalent ? Polar Which Covalent Compounds Are Soluble In Water All nitrates are soluble in water so zn (no 3) 2 is soluble. There are several major types of compounds that are soluble in water. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. This means covalent. Which Covalent Compounds Are Soluble In Water.
From www.britannica.com
covalent bond Definition, Properties, Examples, & Facts Britannica Which Covalent Compounds Are Soluble In Water There are several major types of compounds that are soluble in water. The following sections discuss both soluble and insoluble compounds in water. All bromides are soluble in water, except those combined with pb 2+ so pbbr 2 is insoluble. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. Water is. Which Covalent Compounds Are Soluble In Water.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab Which Covalent Compounds Are Soluble In Water This means that substances with the same type of polarity will be soluble in one another. This means covalent compounds typically don't dissolve in water, instead making a separate layer on the water's surface. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. All nitrates are soluble in water. Which Covalent Compounds Are Soluble In Water.
From sciencenotes.org
Covalent Compounds Examples and Properties Which Covalent Compounds Are Soluble In Water When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. There are several major types of compounds that are soluble in water. Our article on solubility explains how ionic compounds dissolve in water. The following sections discuss both soluble and insoluble compounds in water. All nitrates are soluble in water so zn (no 3) 2. Which Covalent Compounds Are Soluble In Water.
From www.slideserve.com
PPT Bonding PowerPoint Presentation ID3050946 Which Covalent Compounds Are Soluble In Water Water is a polar solvent, but covalent compounds are usually nonpolar. For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. Polar species are soluble in water, while nonpolar species are soluble in oils and. Which Covalent Compounds Are Soluble In Water.
From byjus.com
GIVE REASON SUCROSE IS A COVALENT COMPOUND BUT IT S QUITE SOLUBLE IN WATER? Which Covalent Compounds Are Soluble In Water For instance, the following compounds, all of which are polar and can form hydrogen bonds with water, contain four c. Polar species are soluble in water, while nonpolar species are soluble in oils and fats. When covalent compounds dissolve in water they break apart into molecules, but not individual atoms. All nitrates are soluble in water so zn (no 3). Which Covalent Compounds Are Soluble In Water.