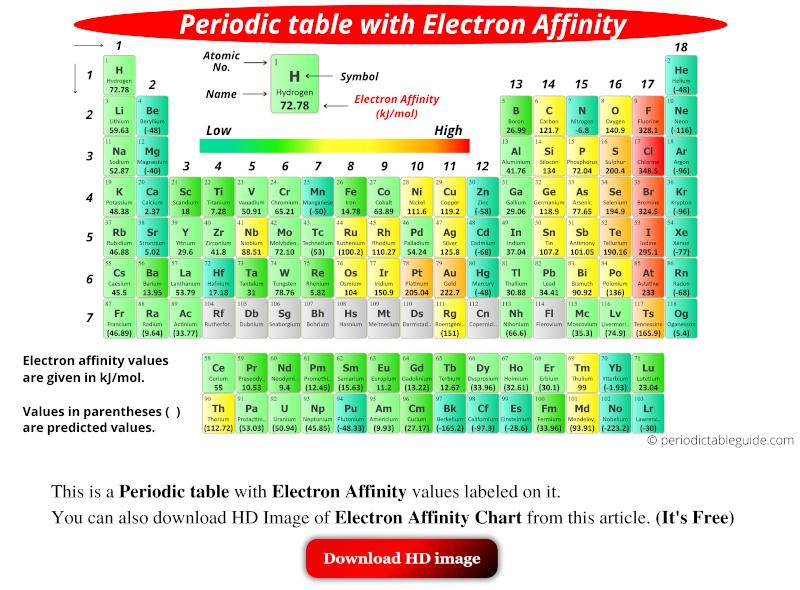
Does Bromine Have More Electron Affinity Than Fluorine . Farther down a column, the attraction for an added electron decreases because the electron is entering an orbital more distant from the nucleus. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. fluorine, therefore, has a lower affinity for an added electron than does chlorine. similarly bromine is a more powerful oxidising agent than iodine. Bromine can remove electrons from iodide ions to give. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). 103 rows table shows electron affinity (i.e. The amount of energy released when an electron is added to atom) for most of. fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of.
from periodictableguide.com
similarly bromine is a more powerful oxidising agent than iodine. Farther down a column, the attraction for an added electron decreases because the electron is entering an orbital more distant from the nucleus. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. 103 rows table shows electron affinity (i.e. Bromine can remove electrons from iodide ions to give. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). fluorine, therefore, has a lower affinity for an added electron than does chlorine. The amount of energy released when an electron is added to atom) for most of.
Electron Affinity Chart (Labeled Periodic table + List)
Does Bromine Have More Electron Affinity Than Fluorine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). fluorine, therefore, has a lower affinity for an added electron than does chlorine. fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. Bromine can remove electrons from iodide ions to give. The amount of energy released when an electron is added to atom) for most of. similarly bromine is a more powerful oxidising agent than iodine. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Farther down a column, the attraction for an added electron decreases because the electron is entering an orbital more distant from the nucleus. 103 rows table shows electron affinity (i.e. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of.
From www.pearson.com
The periodic table with electron affinity values is shown below&... Channels for Pearson+ Does Bromine Have More Electron Affinity Than Fluorine fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). 103 rows table shows electron affinity (i.e. Consequently, the elements of the third row ( n = 3) have the most negative. Does Bromine Have More Electron Affinity Than Fluorine.
From dxogpncsm.blob.core.windows.net
Bromine Element Protons Neutrons And Electrons at Kelly Moya blog Does Bromine Have More Electron Affinity Than Fluorine Farther down a column, the attraction for an added electron decreases because the electron is entering an orbital more distant from the nucleus. Bromine can remove electrons from iodide ions to give. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Consequently, the elements of the third. Does Bromine Have More Electron Affinity Than Fluorine.
From chem.libretexts.org
Electron Affinity Chemistry LibreTexts Does Bromine Have More Electron Affinity Than Fluorine fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. Bromine can remove electrons from iodide ions to give. similarly bromine is a more powerful oxidising agent than iodine. 103 rows table shows electron affinity (i.e. fluorine, therefore, has a lower affinity for an added electron than does chlorine.. Does Bromine Have More Electron Affinity Than Fluorine.
From dxowxzkca.blob.core.windows.net
Fluorine Chlorine Bromine at Alphonse Sparks blog Does Bromine Have More Electron Affinity Than Fluorine similarly bromine is a more powerful oxidising agent than iodine. fluorine, therefore, has a lower affinity for an added electron than does chlorine. Bromine can remove electrons from iodide ions to give. 103 rows table shows electron affinity (i.e. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. . Does Bromine Have More Electron Affinity Than Fluorine.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Does Bromine Have More Electron Affinity Than Fluorine similarly bromine is a more powerful oxidising agent than iodine. The amount of energy released when an electron is added to atom) for most of. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Farther down a column, the attraction for an added electron decreases because the electron is entering an. Does Bromine Have More Electron Affinity Than Fluorine.
From www.pinterest.co.kr
Electron Affinity Trend and Definition Electron affinity, Ionization energy, Electron Does Bromine Have More Electron Affinity Than Fluorine if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). fluorine, therefore, has a lower affinity for an added electron than does chlorine. similarly bromine is a more powerful oxidising agent than iodine. Consequently, the elements of the third row ( n = 3) have the most negative electron. Does Bromine Have More Electron Affinity Than Fluorine.
From www.nuclear-power.com
Fluorine Electron Affinity Electronegativity Ionization Energy of Fluorine Does Bromine Have More Electron Affinity Than Fluorine fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Bromine can remove electrons from iodide ions to give. 103 rows table shows electron affinity (i.e. Farther down a. Does Bromine Have More Electron Affinity Than Fluorine.
From periodictable.thesetupwarrior.com
Electron Affinity Periodic Table Periodic Trends Electron Affinity You Periodic Table Does Bromine Have More Electron Affinity Than Fluorine fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. similarly bromine is a more powerful oxidising agent than iodine. if fluorine gains one more electron, the outermost. Does Bromine Have More Electron Affinity Than Fluorine.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Does Bromine Have More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. 103 rows table shows electron affinity (i.e. Bromine can remove electrons from iodide ions to give. similarly bromine is a more powerful oxidising agent than iodine. The amount of energy released when an electron is added to atom) for most of.. Does Bromine Have More Electron Affinity Than Fluorine.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than chlorine, why does it have a Does Bromine Have More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Bromine can remove electrons from iodide ions to give. fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. electron affinity is the amount of energy change (δe) that occurs when an electron is. Does Bromine Have More Electron Affinity Than Fluorine.
From www.nagwa.com
Question Video Identifying the Explanation for the Anomalous Electron Affinity of Fluorine Nagwa Does Bromine Have More Electron Affinity Than Fluorine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. 103 rows table shows electron affinity (i.e. if fluorine gains one more electron, the outermost p orbitals are completely. Does Bromine Have More Electron Affinity Than Fluorine.
From www.breakingatom.com
Electron Affinity of The Elements Does Bromine Have More Electron Affinity Than Fluorine similarly bromine is a more powerful oxidising agent than iodine. Bromine can remove electrons from iodide ions to give. fluorine, therefore, has a lower affinity for an added electron than does chlorine. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). Farther down a column, the attraction for. Does Bromine Have More Electron Affinity Than Fluorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Does Bromine Have More Electron Affinity Than Fluorine similarly bromine is a more powerful oxidising agent than iodine. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. 103 rows table shows electron affinity (i.e. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. fluorine. Does Bromine Have More Electron Affinity Than Fluorine.
From www.youtube.com
Halogens second electron Affinity is zero Why?Fluorine chlorine bromine iodine have zero 2nd EA Does Bromine Have More Electron Affinity Than Fluorine Farther down a column, the attraction for an added electron decreases because the electron is entering an orbital more distant from the nucleus. 103 rows table shows electron affinity (i.e. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). fluorine is much more reactive than chlorine (despite the. Does Bromine Have More Electron Affinity Than Fluorine.
From www.breakingatom.com
Electron Affinity of The Elements Does Bromine Have More Electron Affinity Than Fluorine Bromine can remove electrons from iodide ions to give. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. electron affinity is the amount of energy change (δe) that occurs when an. Does Bromine Have More Electron Affinity Than Fluorine.
From periodictableguide.com
Electron Affinity Chart (Labeled Periodic table + List) Does Bromine Have More Electron Affinity Than Fluorine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. The amount of energy released when an electron is added to atom) for most of. if fluorine gains one more. Does Bromine Have More Electron Affinity Than Fluorine.
From quizdbpharmacies.z4.web.core.windows.net
Number Of Protons And Neutrons In Fluorine Does Bromine Have More Electron Affinity Than Fluorine if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). 103 rows table shows electron affinity (i.e. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Consequently, the elements of the third row ( n = 3). Does Bromine Have More Electron Affinity Than Fluorine.
From pressbooks.online.ucf.edu
4.4 Ionization energy and Electron Affinity Chemistry Fundamentals Does Bromine Have More Electron Affinity Than Fluorine similarly bromine is a more powerful oxidising agent than iodine. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). fluorine is much more reactive than chlorine. Does Bromine Have More Electron Affinity Than Fluorine.
From material-properties.org
Fluorine Periodic Table and Atomic Properties Does Bromine Have More Electron Affinity Than Fluorine if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). 103 rows table shows electron affinity (i.e. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. Bromine can remove electrons from iodide ions to give. similarly. Does Bromine Have More Electron Affinity Than Fluorine.
From slideplayer.com
Learning Objectives General trends of group 17 elements ppt download Does Bromine Have More Electron Affinity Than Fluorine Farther down a column, the attraction for an added electron decreases because the electron is entering an orbital more distant from the nucleus. fluorine, therefore, has a lower affinity for an added electron than does chlorine. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. electron affinity is the amount. Does Bromine Have More Electron Affinity Than Fluorine.
From www.numerade.com
SOLVED Does chlorine or bromine have a more negative (more favorable) electron affinity? Why Does Bromine Have More Electron Affinity Than Fluorine The amount of energy released when an electron is added to atom) for most of. similarly bromine is a more powerful oxidising agent than iodine. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. electron affinity is the amount of energy change (δe) that occurs when an electron is added. Does Bromine Have More Electron Affinity Than Fluorine.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Does Bromine Have More Electron Affinity Than Fluorine electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. 103 rows table shows electron affinity (i.e. fluorine, therefore, has a lower affinity for an added electron than does chlorine. The amount of energy released when an electron is added to atom) for most of. . Does Bromine Have More Electron Affinity Than Fluorine.
From slideplayer.com
Periodic Properties of Elements ppt download Does Bromine Have More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). The amount of energy released when an electron is added to atom) for most of. Bromine can remove electrons from iodide ions to give.. Does Bromine Have More Electron Affinity Than Fluorine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Chlorine Bromine Iodine? The 5 Does Bromine Have More Electron Affinity Than Fluorine The amount of energy released when an electron is added to atom) for most of. fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. fluorine, therefore, has a lower affinity for an added electron than does chlorine. 103 rows table shows electron affinity (i.e. similarly bromine is a. Does Bromine Have More Electron Affinity Than Fluorine.
From utedzz.blogspot.com
Periodic Table Fluorine Valence Electrons Periodic Table Timeline Does Bromine Have More Electron Affinity Than Fluorine The amount of energy released when an electron is added to atom) for most of. 103 rows table shows electron affinity (i.e. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. . Does Bromine Have More Electron Affinity Than Fluorine.
From www.meritnation.com
Does chlorine have higher electron affinity value than fluorine Chemistry Electrolysis Does Bromine Have More Electron Affinity Than Fluorine fluorine, therefore, has a lower affinity for an added electron than does chlorine. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. fluorine is much more reactive than. Does Bromine Have More Electron Affinity Than Fluorine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Chlorine Bromine Iodine? The 5 Does Bromine Have More Electron Affinity Than Fluorine if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). fluorine, therefore, has a lower affinity for an added electron than does chlorine. similarly bromine is a more powerful oxidising agent than iodine. Farther down a column, the attraction for an added electron decreases because the electron is entering. Does Bromine Have More Electron Affinity Than Fluorine.
From www.pinterest.com
Electron Affinity Electron affinity, Periodic table, Chemistry for kids Does Bromine Have More Electron Affinity Than Fluorine similarly bromine is a more powerful oxidising agent than iodine. Bromine can remove electrons from iodide ions to give. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). The amount of energy released when an electron is added to atom) for most of. electron affinity is the amount. Does Bromine Have More Electron Affinity Than Fluorine.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Table of the Elements Does Bromine Have More Electron Affinity Than Fluorine if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). 103 rows table shows electron affinity (i.e. Farther down a column, the attraction for an added electron decreases because the electron is entering an orbital more distant from the nucleus. fluorine is much more reactive than chlorine (despite the. Does Bromine Have More Electron Affinity Than Fluorine.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Does Bromine Have More Electron Affinity Than Fluorine Farther down a column, the attraction for an added electron decreases because the electron is entering an orbital more distant from the nucleus. The amount of energy released when an electron is added to atom) for most of. 103 rows table shows electron affinity (i.e. Bromine can remove electrons from iodide ions to give. if fluorine gains one. Does Bromine Have More Electron Affinity Than Fluorine.
From material-properties.org
Bromine Periodic Table and Atomic Properties Does Bromine Have More Electron Affinity Than Fluorine similarly bromine is a more powerful oxidising agent than iodine. Bromine can remove electrons from iodide ions to give. fluorine, therefore, has a lower affinity for an added electron than does chlorine. Farther down a column, the attraction for an added electron decreases because the electron is entering an orbital more distant from the nucleus. The amount of. Does Bromine Have More Electron Affinity Than Fluorine.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Does Bromine Have More Electron Affinity Than Fluorine Bromine can remove electrons from iodide ions to give. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. fluorine, therefore, has a lower affinity for an added electron than does chlorine. fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. The amount. Does Bromine Have More Electron Affinity Than Fluorine.
From www.nagwa.com
Question Video Determining the Equation that Represents the Electron Affinity of Fluorine Nagwa Does Bromine Have More Electron Affinity Than Fluorine The amount of energy released when an electron is added to atom) for most of. fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. Farther down a column, the attraction for an added electron. Does Bromine Have More Electron Affinity Than Fluorine.
From www.slideserve.com
PPT Topic Chemistry Aim Explain how elements are classified in the Periodic Table of Does Bromine Have More Electron Affinity Than Fluorine Consequently, the elements of the third row ( n = 3) have the most negative electron affinities. if fluorine gains one more electron, the outermost p orbitals are completely filled (resulting in a full octet). Bromine can remove electrons from iodide ions to give. electron affinity is the amount of energy change (δe) that occurs when an electron. Does Bromine Have More Electron Affinity Than Fluorine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Does Bromine Have More Electron Affinity Than Fluorine fluorine is much more reactive than chlorine (despite the lower electron affinity) because the energy released in. electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of. The amount of energy released when an electron is added to atom) for most of. similarly bromine is a. Does Bromine Have More Electron Affinity Than Fluorine.