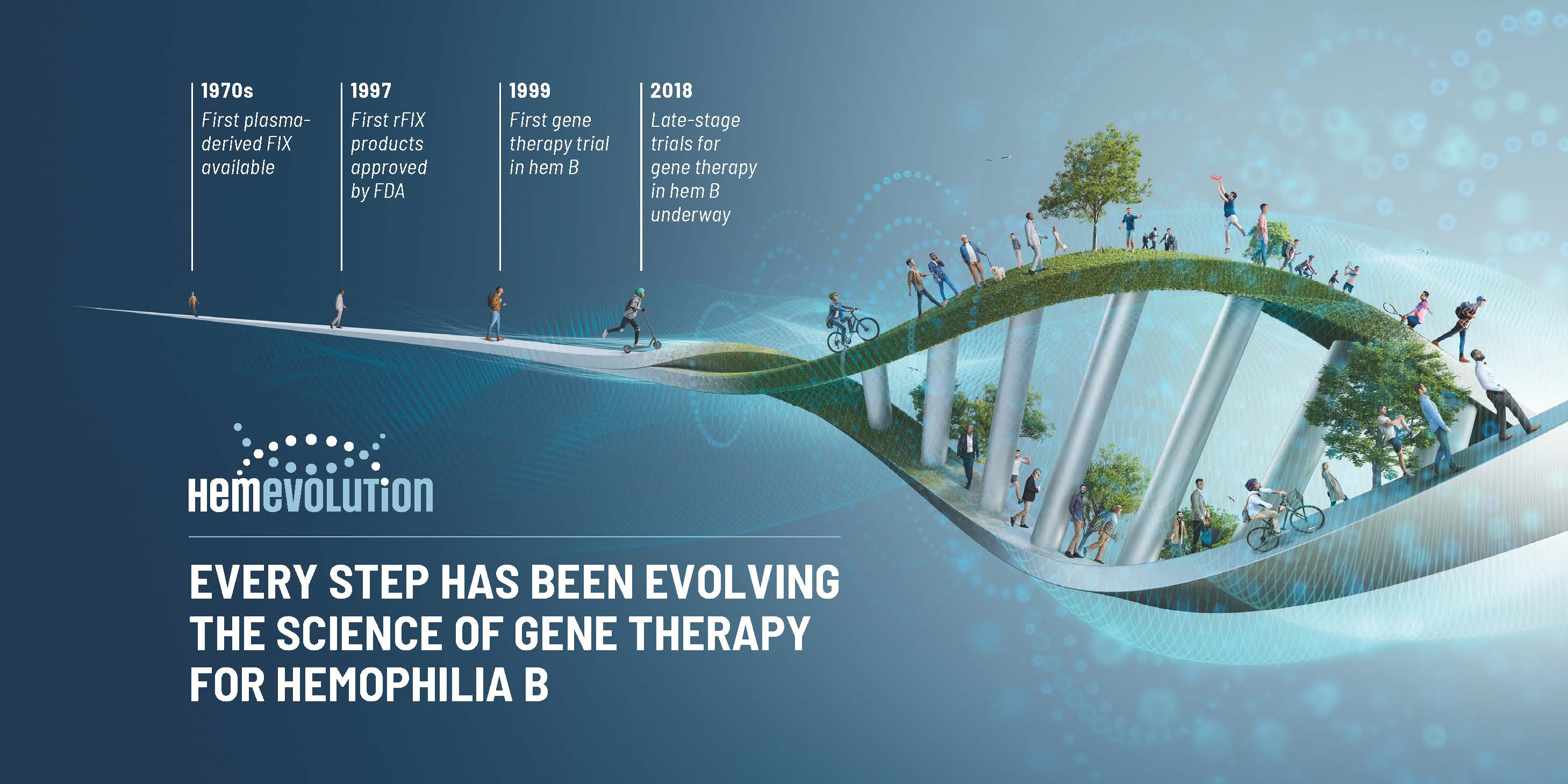
Gene Therapy Hemophilia B . Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Flt180a consists of a synthetic capsid. The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. The gene therapy enables the liver to create factor ix, which allows the blood.
from hemaware.org
Hemophilia b is caused by a lack of clotting factor ix. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Flt180a consists of a synthetic capsid. The gene therapy enables the liver to create factor ix, which allows the blood. The food and drug administration.
Hemophilia B—an excellent candidate for gene therapy HemAware
Gene Therapy Hemophilia B The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Flt180a consists of a synthetic capsid. The gene therapy enables the liver to create factor ix, which allows the blood. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement.
From www.semanticscholar.org
Figure 3 from Hemophilia B Gene Therapy with a High‐Specific‐Activity Gene Therapy Hemophilia B Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. Gene Therapy Hemophilia B.
From www.cell.com
A Molecular Revolution in the Treatment of Hemophilia Molecular Therapy Gene Therapy Hemophilia B The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. Gene Therapy Hemophilia B.
From hemaware.org
Hemophilia B—an excellent candidate for gene therapy HemAware Gene Therapy Hemophilia B Flt180a consists of a synthetic capsid. Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Gene Therapy Hemophilia B.
From planningshopintl.com
Gene therapy and Haemophilia THE PLANNING SHOP Gene Therapy Hemophilia B The gene therapy enables the liver to create factor ix, which allows the blood. Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Gene Therapy Hemophilia B.
From www.youtube.com
Gene Therapy An End to Hemophilia YouTube Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Gene Therapy Hemophilia B.
From journals.lww.com
Hemophilia Gene Therapy Approaching the First Licensed Prod Gene Therapy Hemophilia B The gene therapy enables the liver to create factor ix, which allows the blood. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Flt180a consists of a synthetic capsid. Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Gene Therapy Hemophilia B.
From www.youtube.com
Recent Advances in Gene Therapy for Hemophilia Presented at NHF 2021 Gene Therapy Hemophilia B The gene therapy enables the liver to create factor ix, which allows the blood. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From www.genengnews.com
Hemophilia B Gene Therapy Shows Promise in Clinical Trial Gene Therapy Hemophilia B Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. The gene therapy enables the liver to create factor ix, which allows the blood. Gene Therapy Hemophilia B.
From www.cell.com
PlateletTargeted Gene Therapy for Hemophilia Molecular Therapy Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. The gene therapy enables the liver to create factor ix, which allows the blood. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Flt180a consists of a synthetic capsid. The food and drug administration. Gene Therapy Hemophilia B.
From www.quanticate.com
FDA Guidance for Human Gene Therapy for Hemophilia A & B Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From www.hematologyadvisor.com
Expert Q&A The Current State of Gene Therapy for Hemophilia Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From planningshopintl.com
Gene therapy and Haemophilia The Planning Shop Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. The gene therapy enables the liver to create factor ix, which allows the blood. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From www.regmednet.com
Data from clinical trial of gene therapy for hemophilia B Gene Therapy Hemophilia B The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Gene Therapy Hemophilia B.
From healthmatch.io
HealthMatch FDA Approves First Gene Therapy To Treat Adults With Gene Therapy Hemophilia B Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From www.slideserve.com
PPT AAV Gene Therapies for Hemophilia B Treatment_ The Road to a Cure Gene Therapy Hemophilia B The gene therapy enables the liver to create factor ix, which allows the blood. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From hemophilianewstoday.com
Hemophilia B Treated in Mice Using Gene Therapy Delivered by Microbubbles Gene Therapy Hemophilia B The food and drug administration. Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Hemophilia b is caused by a lack of clotting factor ix. Gene Therapy Hemophilia B.
From www.youtube.com
Gene Therapy Basics for Hemophilia What You Need to Know YouTube Gene Therapy Hemophilia B Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From animalia-life.club
Gene Therapy Hemophilia Gene Therapy Hemophilia B Flt180a consists of a synthetic capsid. The gene therapy enables the liver to create factor ix, which allows the blood. The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Gene Therapy Hemophilia B.
From reviopharma.com
EMA starts review of the first gene therapy to treat Hemophilia B Gene Therapy Hemophilia B The food and drug administration. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Hemophilia b is caused by a lack of clotting factor ix. Gene Therapy Hemophilia B.
From www.trasci.com
Gene therapy for hemophilia Progress to date and challenges moving Gene Therapy Hemophilia B The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Flt180a consists of a synthetic capsid. The gene therapy enables the liver to create factor ix, which allows the blood. Gene Therapy Hemophilia B.
From www.researchgate.net
Hemophilia B gene therapy with AAV vectors Download Table Gene Therapy Hemophilia B The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. Gene Therapy Hemophilia B.
From www.newscientist.com
Haemophilia B Gene therapy trial shows early success in people with Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The food and drug administration. Gene Therapy Hemophilia B.
From www.haemnet.com
Gene therapy for haemophilia Gene Therapy Hemophilia B Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Hemophilia b is caused by a lack of clotting factor ix. Flt180a consists of a synthetic capsid. The food and drug administration. Gene Therapy Hemophilia B.
From medicalxpress.com
Gene therapy for hemophilia A shows promise in phase 3 clinical trial Gene Therapy Hemophilia B The food and drug administration. Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Hemophilia b is caused by a lack of clotting factor ix. Gene Therapy Hemophilia B.
From www.futureofpersonalhealth.com
Gene Therapy for Treating Hemophilia B Future of Personal Health Gene Therapy Hemophilia B The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From www.nejm.org
Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B NEJM Gene Therapy Hemophilia B The food and drug administration. The gene therapy enables the liver to create factor ix, which allows the blood. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Hemophilia b is caused by a lack of clotting factor ix. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From directorsblog.nih.gov
Encouraging News on Gene Therapy for Hemophilia A NIH Director's Blog Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. Flt180a consists of a synthetic capsid. The gene therapy enables the liver to create factor ix, which allows the blood. The food and drug administration. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Gene Therapy Hemophilia B.
From www.cell.com
FIX It in One Go Enhanced Factor IX Gene Therapy for Hemophilia B Cell Gene Therapy Hemophilia B Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. The food and drug administration. Hemophilia b is caused by a lack of clotting factor ix. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From www.europeanpharmaceuticalreview.com
FDA approves first gene therapy for Haemophilia B Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. The food and drug administration. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From ar.inspiredpencil.com
Gene Therapy Hemophilia Gene Therapy Hemophilia B Flt180a consists of a synthetic capsid. The gene therapy enables the liver to create factor ix, which allows the blood. Hemophilia b is caused by a lack of clotting factor ix. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The food and drug administration. Gene Therapy Hemophilia B.
From www.creative-biolabs.com
Gene Therapy for Hemophilia B Using Viral and NonViral Vectors Gene Therapy Hemophilia B The food and drug administration. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Hemophilia b is caused by a lack of clotting factor ix. Gene Therapy Hemophilia B.
From elearning.wfh.org
Gene Therapy for Hemophilia B Fact Sheet eLearning Platform Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene Therapy Hemophilia B.
From www.labiotech.eu
First Potential Gene Therapy for Hemophilia B Restores Blood Clotting Gene Therapy Hemophilia B Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Flt180a consists of a synthetic capsid. The gene therapy enables the liver to create factor ix, which allows the blood. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Gene Therapy Hemophilia B.
From medicaldialogues.in
First gene therapy for hemophilia B treatment receives FDA approval Gene Therapy Hemophilia B The gene therapy enables the liver to create factor ix, which allows the blood. Flt180a consists of a synthetic capsid. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Hemophilia b is caused by a lack of clotting factor ix. The food and drug administration. Gene Therapy Hemophilia B.
From www.empr.com
Etranacogene Dezaparvovec, a Gene Therapy for Hemophilia B, Gets Gene Therapy Hemophilia B The gene therapy enables the liver to create factor ix, which allows the blood. The food and drug administration. Gene therapy for hemophilia b aims to establish sustained factor ix activity, thereby protecting against bleeding without burdensome factor ix replacement. Flt180a consists of a synthetic capsid. Hemophilia b is caused by a lack of clotting factor ix. Gene Therapy Hemophilia B.