Experiments With Zinc . copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. you can weigh the coins before and after coating to find the mass of zinc added. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. updated on february 03, 2020. using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. There are many interesting chemistry projects you can do using metals and alloys. This gives a gold appearance to the coin. Zinc powder is added to a solution of iodine in ethanol. This can be reversed using electrolysis to decompose the compound. Brass is an alloy of copper containing between 18% and 40% of zinc. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can
from sci-toys.com
There are many interesting chemistry projects you can do using metals and alloys. using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. updated on february 03, 2020. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can Brass is an alloy of copper containing between 18% and 40% of zinc. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Zinc powder is added to a solution of iodine in ethanol. you can weigh the coins before and after coating to find the mass of zinc added.
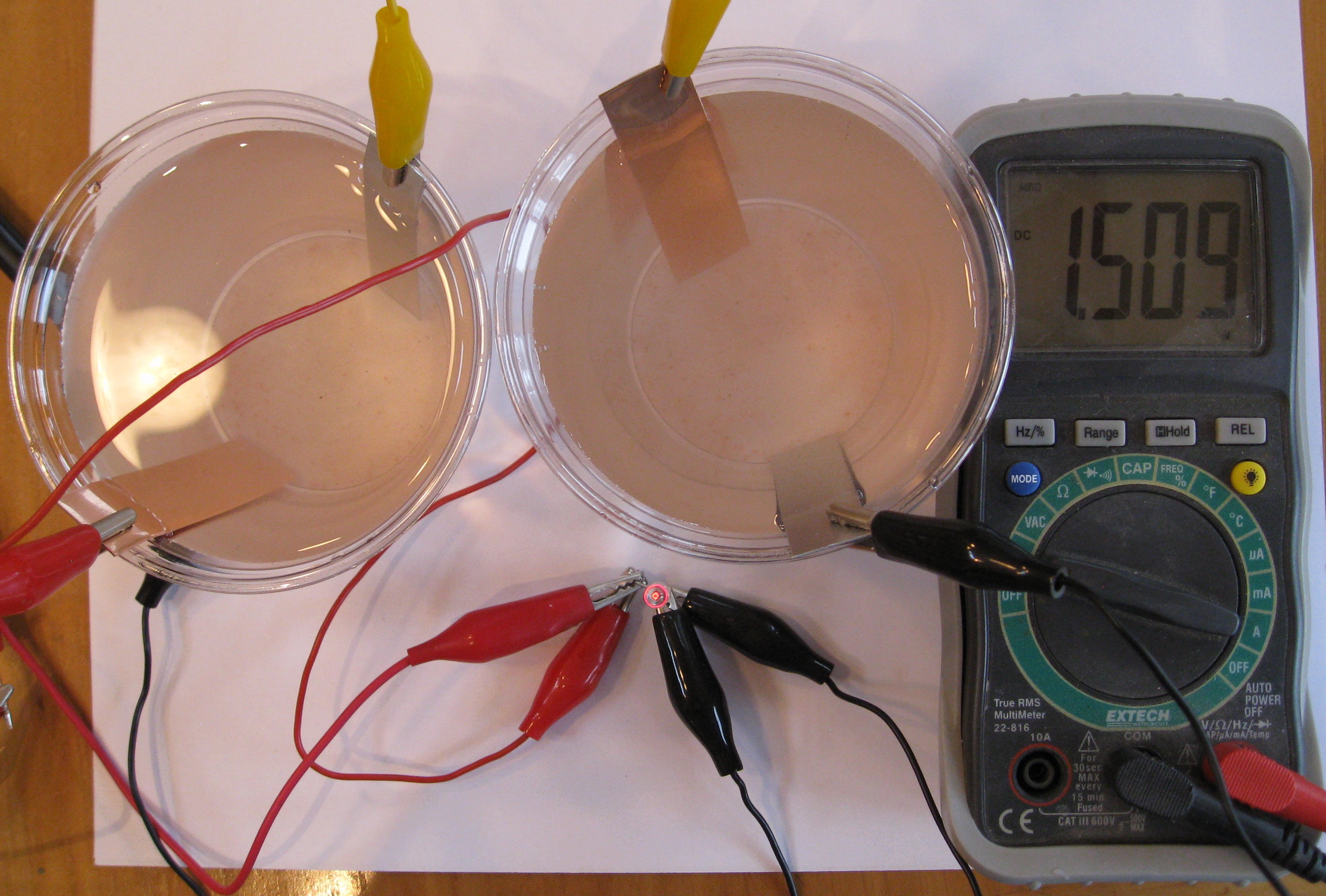
Chapter 3 Electrochemistry Make homemade batteries in your kitchen
Experiments With Zinc There are many interesting chemistry projects you can do using metals and alloys. On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. Zinc powder is added to a solution of iodine in ethanol. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can Brass is an alloy of copper containing between 18% and 40% of zinc. you can weigh the coins before and after coating to find the mass of zinc added. This can be reversed using electrolysis to decompose the compound. This gives a gold appearance to the coin. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. There are many interesting chemistry projects you can do using metals and alloys. updated on february 03, 2020. using an exothermic redox reaction between zinc and iodine, student will make zinc iodide.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0146 Science Experiments With Zinc On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. This can be reversed using electrolysis to decompose the compound. you can weigh the coins before and after coating to find the mass of zinc added. copper(ii) oxide and zinc metal react together in an exothermic. Experiments With Zinc.
From melscience.com
Zinccarbon battery MEL Chemistry Experiments With Zinc By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can updated on february 03, 2020. using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. On heating. Experiments With Zinc.
From flatworldknowledge.lardbucket.org
Electrochemistry Experiments With Zinc Brass is an alloy of copper containing between 18% and 40% of zinc. you can weigh the coins before and after coating to find the mass of zinc added. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. This can be reversed using electrolysis to decompose the compound. using. Experiments With Zinc.
From miniaturedreams.blogspot.com
Miniature Dreams Experiments in zinc Experiments With Zinc Brass is an alloy of copper containing between 18% and 40% of zinc. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. Zinc powder is added to a solution of iodine in ethanol. This gives a gold appearance to the coin. On heating the coin in the bunsen flame, brass is formed by the zinc. Experiments With Zinc.
From www.youtube.com
Beautiful Chemical reaction between zinc and Iodine Chemistry Experiments With Zinc this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. you can weigh the coins before and after coating to find the mass of zinc added. Zinc powder is added to a solution of iodine in ethanol. using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. On heating the. Experiments With Zinc.
From www.inceptivemind.com
Researchers develop aqueous zincion battery that uses water as an Experiments With Zinc you can weigh the coins before and after coating to find the mass of zinc added. On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. This gives a gold appearance to the. Experiments With Zinc.
From miniaturedreams.blogspot.com
Miniature Dreams Experiments in zinc Experiments With Zinc Zinc powder is added to a solution of iodine in ethanol. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. you can weigh the coins before and after coating to find the mass of zinc. Experiments With Zinc.
From edu.rsc.org
Catalysis of the reaction between zinc and sulfuric acid Experiment Experiments With Zinc This can be reversed using electrolysis to decompose the compound. This gives a gold appearance to the coin. updated on february 03, 2020. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. There are many interesting chemistry projects you can do using metals and alloys. using an exothermic redox. Experiments With Zinc.
From www.alamy.com
lowcost experiment, voltaic cell with a copper strip and zinc strip Experiments With Zinc Zinc powder is added to a solution of iodine in ethanol. On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. This can be reversed using electrolysis to decompose the compound. updated on february 03, 2020. There are many interesting chemistry projects you can do using metals. Experiments With Zinc.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER Experiments With Zinc copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. This can be reversed using electrolysis to decompose the compound. This gives a gold appearance to the coin. you can weigh the coins before and after. Experiments With Zinc.
From www.youtube.com
Zinc Reacts with Hot Water Grade 8 Science Experiments Easy Science Experiments With Zinc copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Zinc powder is added to a solution of iodine in ethanol. There are many interesting chemistry projects you can do using metals and alloys. This gives a gold appearance to the coin. By observing this reaction and its products, and noting the. Experiments With Zinc.
From www.pinterest.co.uk
Super Science Fair Projects PicoSolutions Kearny, NJ Science fair Experiments With Zinc this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. This can be reversed using electrolysis to decompose the compound. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. Brass is an alloy of copper containing between 18% and 40% of zinc. There are many interesting chemistry. Experiments With Zinc.
From www.youtube.com
Preparing Zinc Oxide Nanoparticles YouTube Experiments With Zinc this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. There are many interesting chemistry projects you can do using metals and alloys. Brass is an alloy of copper containing between 18% and 40% of zinc. On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of. Experiments With Zinc.
From schneidersci.blogspot.com
Schneider Science Zinc & Hydrochloric Acid Experiments With Zinc There are many interesting chemistry projects you can do using metals and alloys. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. updated on february 03, 2020. Brass is an alloy of copper containing between 18% and 40% of zinc. This gives a gold appearance to the coin. On heating. Experiments With Zinc.
From communityhigh.net
ZnCl₂ Formula » Community High School Experiments With Zinc Zinc powder is added to a solution of iodine in ethanol. There are many interesting chemistry projects you can do using metals and alloys. you can weigh the coins before and after coating to find the mass of zinc added. This can be reversed using electrolysis to decompose the compound. By observing this reaction and its products, and noting. Experiments With Zinc.
From www.thoughtco.com
2 Easy Ways to Get Zinc Metal Experiments With Zinc On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. updated on february 03, 2020. There are many interesting chemistry projects you can do using metals and alloys. you can weigh the coins before and after coating to find the mass of zinc added. By observing. Experiments With Zinc.
From www.alamy.com
a smoke from burning zinc, chemical experiments Stock Photo Alamy Experiments With Zinc Brass is an alloy of copper containing between 18% and 40% of zinc. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can There are many interesting chemistry projects you can do using metals and alloys. updated on february 03, 2020. you can weigh the coins before and after. Experiments With Zinc.
From melscience.com
Zinc plating MEL Chemistry Experiments With Zinc this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can This can be reversed using electrolysis to decompose the compound. using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Zinc powder. Experiments With Zinc.
From www.pinterest.com
DIY Enviro Battery Model Generator Zinc Copper Fruit Potato Battery Experiments With Zinc By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. This gives a gold appearance to the coin. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. This can be reversed. Experiments With Zinc.
From www.researchgate.net
Zinc isotope compositions for sorption edge experiments ([Zn] ini. = 50 Experiments With Zinc This can be reversed using electrolysis to decompose the compound. Zinc powder is added to a solution of iodine in ethanol. This gives a gold appearance to the coin. updated on february 03, 2020. Brass is an alloy of copper containing between 18% and 40% of zinc. using an exothermic redox reaction between zinc and iodine, student will. Experiments With Zinc.
From science.wonderhowto.com
How to Make solid rocket fuel ignition with zinc and sulfur « Science Experiments With Zinc using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Zinc powder is added to a solution of iodine in ethanol. There are many interesting chemistry projects you can do using metals and alloys. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can you. Experiments With Zinc.
From saylordotorg.github.io
Electrochemistry Experiments With Zinc using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. This can be reversed using electrolysis to decompose the compound. you can weigh the coins before and after coating to find the mass of zinc added. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper.. Experiments With Zinc.
From www.numerade.com
SOLVED Question 2 A student carried out some experiments between zinc Experiments With Zinc On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. Brass is an alloy of copper containing between 18% and 40% of zinc. This gives a gold appearance to the coin. updated on february 03, 2020. this review summarizes representative examples of zinc’s action on bacterial. Experiments With Zinc.
From www.youtube.com
zinc oxide powder and eugenol mixing technique YouTube Experiments With Zinc There are many interesting chemistry projects you can do using metals and alloys. Brass is an alloy of copper containing between 18% and 40% of zinc. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. On heating the coin in the bunsen flame, brass is formed by the zinc migrating into. Experiments With Zinc.
From www.researchgate.net
Results of zinc removal experiments dosing synthetic zinc sulphate Experiments With Zinc By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can Brass is an alloy of copper containing between 18% and 40% of zinc. There are many interesting chemistry projects you can do using metals and alloys. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. This. Experiments With Zinc.
From www.wikihow.com
How to Get Zinc Metal 11 Steps (with Pictures) wikiHow Experiments With Zinc Brass is an alloy of copper containing between 18% and 40% of zinc. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. On heating the coin in the bunsen flame, brass is formed by the. Experiments With Zinc.
From www.chegg.com
Solved Chemical formula Experiments (A) zinc chloride 1. Experiments With Zinc Brass is an alloy of copper containing between 18% and 40% of zinc. Zinc powder is added to a solution of iodine in ethanol. updated on february 03, 2020. This gives a gold appearance to the coin. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. By observing this reaction and its products, and. Experiments With Zinc.
From www.thoughtco.com
Two Easy Ways to Get Zinc Metal Experiments With Zinc Zinc powder is added to a solution of iodine in ethanol. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. This can be reversed using electrolysis to decompose the compound. updated on february 03, 2020. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. . Experiments With Zinc.
From www.youtube.com
3 experiments with copper zinc plates Amazing hacks YouTube Experiments With Zinc There are many interesting chemistry projects you can do using metals and alloys. By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can Zinc powder is added to a solution of iodine in ethanol. Brass is an alloy of copper containing between 18% and 40% of zinc. This gives a gold. Experiments With Zinc.
From www.youtube.com
The action of heat on zinc II nitrate Laboratory experiments on salts Experiments With Zinc updated on february 03, 2020. copper(ii) oxide and zinc metal react together in an exothermic reaction to produce zinc oxide and copper. This gives a gold appearance to the coin. Brass is an alloy of copper containing between 18% and 40% of zinc. By observing this reaction and its products, and noting the difference in reactivity between zinc. Experiments With Zinc.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Experiments With Zinc This can be reversed using electrolysis to decompose the compound. On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. There are many interesting chemistry projects you can do using metals and alloys. updated on february 03, 2020. this review summarizes representative examples of zinc’s action. Experiments With Zinc.
From www.researchgate.net
(PDF) Notes on Experiments on the Joining of Zinc Castings Experiments With Zinc By observing this reaction and its products, and noting the difference in reactivity between zinc and copper, students can updated on february 03, 2020. There are many interesting chemistry projects you can do using metals and alloys. On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper.. Experiments With Zinc.
From sci-toys.com
Chapter 3 Electrochemistry Make homemade batteries in your kitchen Experiments With Zinc you can weigh the coins before and after coating to find the mass of zinc added. Brass is an alloy of copper containing between 18% and 40% of zinc. using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. By observing this reaction and its products, and noting the difference in reactivity between zinc. Experiments With Zinc.
From labasiabd.com
Zinc Plate for Electrochemistry Experiments Lab Asia Experiments With Zinc This can be reversed using electrolysis to decompose the compound. Zinc powder is added to a solution of iodine in ethanol. this review summarizes representative examples of zinc’s action on bacterial virulence and antibiotic. There are many interesting chemistry projects you can do using metals and alloys. updated on february 03, 2020. On heating the coin in the. Experiments With Zinc.
From www.techeblog.com
Scientists Grow Microscopic Zinc Snowflakes in Liquid Metal at the Experiments With Zinc On heating the coin in the bunsen flame, brass is formed by the zinc migrating into the surface layer of the copper. This gives a gold appearance to the coin. Zinc powder is added to a solution of iodine in ethanol. using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. updated on february. Experiments With Zinc.