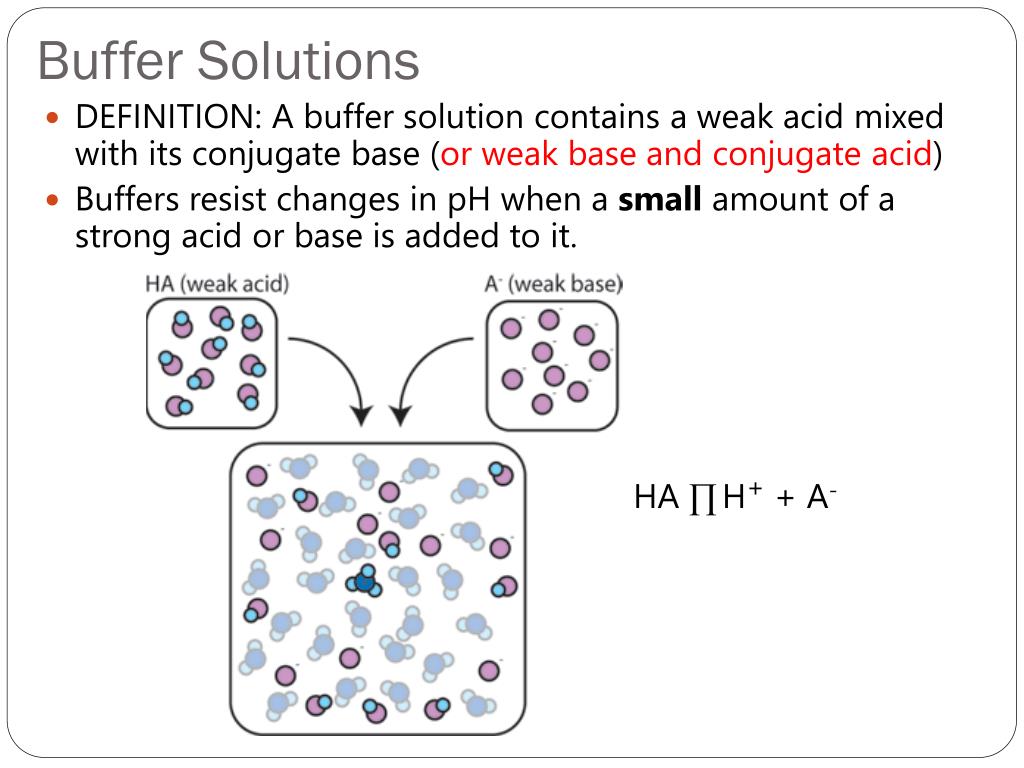
What Happens When A Base Is Added To A Buffer . a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added.
from www.slideserve.com
the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. the buffering action of the solution is essentially a result of the added strong acid and base being converted to.
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242
What Happens When A Base Is Added To A Buffer buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens When A Base Is Added To A Buffer a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. the buffer capacity is the amount of acid or base that can be. What Happens When A Base Is Added To A Buffer.
From www.chegg.com
Solved When an acid or a base is added to a buffer, What Happens When A Base Is Added To A Buffer if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. buffer solutions resist a change in ph when small amounts of a strong acid or a strong. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT Unit 18 AcidBase Equilibria Buffers & Hydrolysis PowerPoint What Happens When A Base Is Added To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. the buffer capacity is the amount of acid or base that can be added to. What Happens When A Base Is Added To A Buffer.
From www.chegg.com
Solved When an acid or a base is added to a buffer, What Happens When A Base Is Added To A Buffer a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. if we add a base (hydroxide ions), ammonium ions in the buffer react with the. What Happens When A Base Is Added To A Buffer.
From design.udlvirtual.edu.pe
What Is A Buffer Solution Design Talk What Happens When A Base Is Added To A Buffer buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. A buffer solution is one which resists changes in ph when small. What Happens When A Base Is Added To A Buffer.
From chem.libretexts.org
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts What Happens When A Base Is Added To A Buffer buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. the buffering action of the solution is essentially a result of the added strong acid and base. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download What Happens When A Base Is Added To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. the buffer capacity is the amount of acid or base that can be added to. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint What Happens When A Base Is Added To A Buffer if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. A buffer solution is one which resists changes in ph when small. What Happens When A Base Is Added To A Buffer.
From sciencing.com
What Happens When a Base Is Added to a Buffer Solution? Sciencing What Happens When A Base Is Added To A Buffer a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffering action of the solution is essentially a. What Happens When A Base Is Added To A Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Happens When A Base Is Added To A Buffer if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. buffer solutions resist a change in ph when small amounts of a strong acid or a strong. What Happens When A Base Is Added To A Buffer.
From www.numerade.com
SOLVED A buffer contains significant amounts of ammonia and ammonium What Happens When A Base Is Added To A Buffer the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. the buffering action of the solution is essentially a. What Happens When A Base Is Added To A Buffer.
From byjus.com
When a small amount of HCL is added to a buffer solution of acetic acid What Happens When A Base Is Added To A Buffer buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. if we add a base (hydroxide ions), ammonium ions in the. What Happens When A Base Is Added To A Buffer.
From www.chegg.com
Solved If a base is added to a buffered solution, what must What Happens When A Base Is Added To A Buffer buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. if we add a base (hydroxide ions), ammonium ions in the buffer react with the. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT What is a buffer? PowerPoint Presentation, free download ID1149749 What Happens When A Base Is Added To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. a buffer that contains approximately equal amounts of a weak acid and its conjugate base. What Happens When A Base Is Added To A Buffer.
From slideplayer.com
PH and Buffers. ppt download What Happens When A Base Is Added To A Buffer a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. A buffer solution is one which resists changes in ph when small quantities of an acid. What Happens When A Base Is Added To A Buffer.
From www.youtube.com
26 Acid Base Buffers YouTube What Happens When A Base Is Added To A Buffer buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer that contains approximately equal amounts of a weak acid and its conjugate base. What Happens When A Base Is Added To A Buffer.
From courses.lumenlearning.com
Buffers Chemistry for Majors What Happens When A Base Is Added To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. if we add a base (hydroxide ions), ammonium ions in the buffer react with the. What Happens When A Base Is Added To A Buffer.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Happens When A Base Is Added To A Buffer the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. buffer solutions resist a change in ph when small. What Happens When A Base Is Added To A Buffer.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Happens When A Base Is Added To A Buffer if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. A buffer solution is one which resists changes in ph when small quantities of an acid or an. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT Acids Bases Equilibria Part V Buffers PowerPoint Presentation What Happens When A Base Is Added To A Buffer if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. the buffering action of the solution is essentially a result of the added strong acid and base. What Happens When A Base Is Added To A Buffer.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Happens When A Base Is Added To A Buffer the buffering action of the solution is essentially a result of the added strong acid and base being converted to. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. a buffer that contains approximately equal amounts of a weak acid. What Happens When A Base Is Added To A Buffer.
From www.youtube.com
AP chemistry Adding Strong Acid to Buffer problem YouTube What Happens When A Base Is Added To A Buffer the buffering action of the solution is essentially a result of the added strong acid and base being converted to. a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. A buffer solution is one which resists changes in ph when small quantities of an acid. What Happens When A Base Is Added To A Buffer.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts What Happens When A Base Is Added To A Buffer if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. A buffer solution is one which resists changes in ph when small quantities of an acid or an. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free What Happens When A Base Is Added To A Buffer the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. buffer solutions resist a change in ph when small amounts of. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download What Happens When A Base Is Added To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. buffer solutions resist a change in ph when small amounts of a strong acid or. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 What Happens When A Base Is Added To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. if we add a base (hydroxide ions), ammonium ions. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens When A Base Is Added To A Buffer a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added. if we add a base (hydroxide ions), ammonium ions in the buffer react with the. What Happens When A Base Is Added To A Buffer.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Happens When A Base Is Added To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. buffer solutions resist a change in ph when small amounts of a strong. What Happens When A Base Is Added To A Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens When A Base Is Added To A Buffer a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. if we add a base (hydroxide ions), ammonium ions. What Happens When A Base Is Added To A Buffer.
From www.chegg.com
Solved If a base is added to a buffered solution, what must What Happens When A Base Is Added To A Buffer the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer that contains approximately equal amounts of a. What Happens When A Base Is Added To A Buffer.
From www.youtube.com
Adding strong base to a buffer solution YouTube What Happens When A Base Is Added To A Buffer if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. the buffer capacity is the amount of acid or base that can be added to a given. What Happens When A Base Is Added To A Buffer.
From www.youtube.com
Adding Acid to a Buffer Calculating the pH using Henderson What Happens When A Base Is Added To A Buffer the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. a buffer that contains approximately equal amounts of a weak acid. What Happens When A Base Is Added To A Buffer.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content What Happens When A Base Is Added To A Buffer the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffering action of the solution is essentially a result of the added strong acid and base being converted to. if we add a base (hydroxide ions), ammonium ions in the. What Happens When A Base Is Added To A Buffer.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide What Happens When A Base Is Added To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. the buffering action of the solution is essentially a result of the added. What Happens When A Base Is Added To A Buffer.
From www.chegg.com
Solved When an acid or a base is added to a buffer, a What Happens When A Base Is Added To A Buffer if we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia. a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at neutralizing. A buffer solution is one which resists changes in ph when small quantities of an acid. What Happens When A Base Is Added To A Buffer.