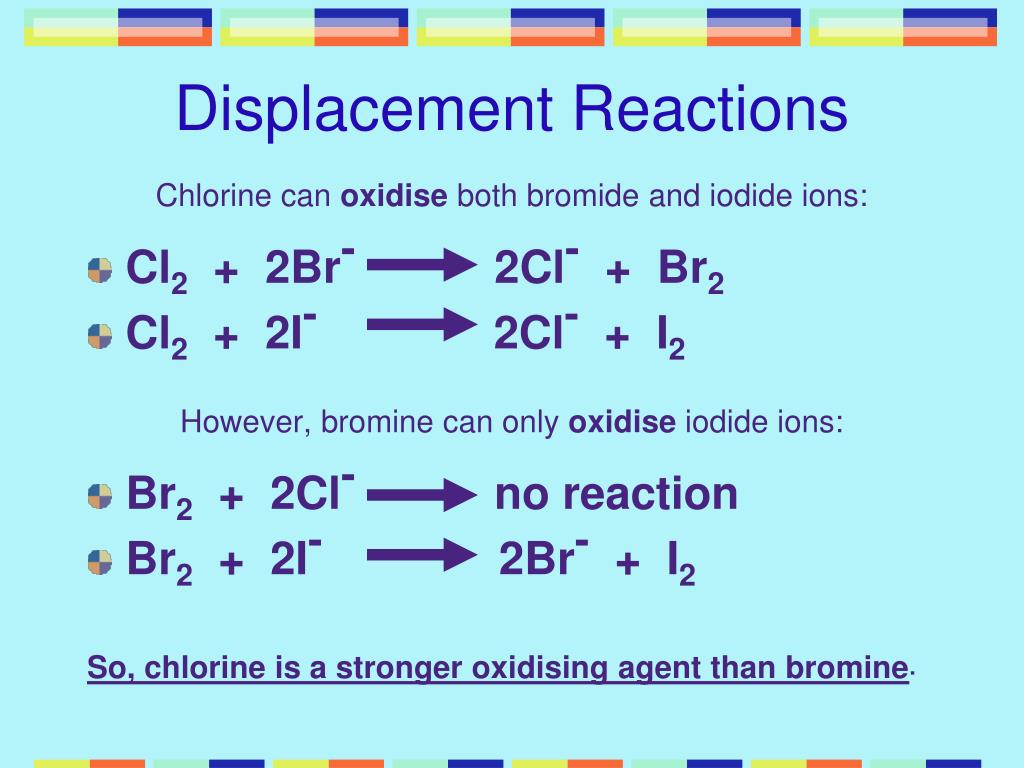
Reaction Of Chlorine Bromine And Iodine With Other Halide Ions . when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. a solution of chlorine can displace iodine from potassium iodide solution: discussions of the chemistry of the elements in group viia therefore focus on four elements: C iodine will displace fluorine from lithium fluoride solution. the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. Chlorine + potassium iodide → potassium chloride +. b chlorine will displace bromine from sodium bromide solution. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the.
from www.slideserve.com
C iodine will displace fluorine from lithium fluoride solution. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. a solution of chlorine can displace iodine from potassium iodide solution: the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. Chlorine + potassium iodide → potassium chloride +. b chlorine will displace bromine from sodium bromide solution. discussions of the chemistry of the elements in group viia therefore focus on four elements:
PPT Halogens PowerPoint Presentation, free download ID2110165
Reaction Of Chlorine Bromine And Iodine With Other Halide Ions discussions of the chemistry of the elements in group viia therefore focus on four elements: when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. C iodine will displace fluorine from lithium fluoride solution. a solution of chlorine can displace iodine from potassium iodide solution: Chlorine + potassium iodide → potassium chloride +. b chlorine will displace bromine from sodium bromide solution. discussions of the chemistry of the elements in group viia therefore focus on four elements: the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Reaction Of Chlorine Bromine And Iodine With Other Halide Ions when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Chlorine + potassium iodide → potassium chloride +. C iodine will displace fluorine from lithium fluoride solution. discussions of the chemistry of the elements in group viia therefore focus on four elements: revision notes on. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From kpu.pressbooks.pub
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Reaction Of Chlorine Bromine And Iodine With Other Halide Ions C iodine will displace fluorine from lithium fluoride solution. the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. a solution of chlorine can displace iodine from potassium iodide solution: b chlorine will displace bromine from sodium bromide solution. discussions of the chemistry of the elements in group. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideserve.com
PPT iGCSE chemistry Section 2 lesson 2 PowerPoint Presentation, free Reaction Of Chlorine Bromine And Iodine With Other Halide Ions Chlorine + potassium iodide → potassium chloride +. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. discussions of the chemistry of the elements in group viia therefore focus on four elements: C iodine will displace fluorine from lithium fluoride solution. a solution of. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes Master Organic Chemistry Reaction Of Chlorine Bromine And Iodine With Other Halide Ions Chlorine + potassium iodide → potassium chloride +. b chlorine will displace bromine from sodium bromide solution. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. . Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From edu.rsc.org
Reactions of chlorine, bromine and iodine with aluminium Experiment Reaction Of Chlorine Bromine And Iodine With Other Halide Ions b chlorine will displace bromine from sodium bromide solution. the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. a solution of chlorine can displace iodine. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From slideplayer.com
Reactions of the halogens and halide ions ppt download Reaction Of Chlorine Bromine And Iodine With Other Halide Ions the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. a solution of chlorine can displace iodine from potassium iodide solution: C iodine will displace fluorine from lithium fluoride solution. Chlorine + potassium iodide → potassium chloride +. when chlorine (as a gas or dissolved in water) is added. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID8800875 Reaction Of Chlorine Bromine And Iodine With Other Halide Ions discussions of the chemistry of the elements in group viia therefore focus on four elements: the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. C iodine will displace fluorine from lithium fluoride solution. b chlorine will displace bromine from sodium bromide solution. a solution of chlorine can. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.youtube.com
TEST FOR HALIDE IONS (Chloride, Bromide and Iodide ions) 3D animation Reaction Of Chlorine Bromine And Iodine With Other Halide Ions the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. b chlorine will displace bromine from sodium bromide solution. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. discussions of the chemistry of the elements. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Reaction Of Chlorine Bromine And Iodine With Other Halide Ions a solution of chlorine can displace iodine from potassium iodide solution: discussions of the chemistry of the elements in group viia therefore focus on four elements: when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. C iodine will displace fluorine from lithium fluoride solution.. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideshare.net
Oxidation & reduction Reaction Of Chlorine Bromine And Iodine With Other Halide Ions revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. b chlorine will displace bromine from sodium bromide solution. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. a solution of chlorine can displace iodine from. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideshare.net
Lesson 2 Halide Halogen Displacement Reactions. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions a solution of chlorine can displace iodine from potassium iodide solution: when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. b chlorine will displace bromine from sodium bromide solution. the relative oxidising strengths of the halogens can be seen by their displacement reactions. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideserve.com
PPT Precipitate Reactions PowerPoint Presentation, free download ID Reaction Of Chlorine Bromine And Iodine With Other Halide Ions b chlorine will displace bromine from sodium bromide solution. the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. C iodine will displace fluorine from lithium fluoride solution. a solution of chlorine can displace iodine from potassium iodide solution: discussions of the chemistry of the elements in group. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.savemyexams.com
Testing for Halide Ions AQA A Level Chemistry Revision Notes 2017 Reaction Of Chlorine Bromine And Iodine With Other Halide Ions a solution of chlorine can displace iodine from potassium iodide solution: the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. C iodine will displace fluorine from lithium fluoride solution. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.numerade.com
SOLVED 1.Chlorine bromine and iodine all react with alkenes such as Reaction Of Chlorine Bromine And Iodine With Other Halide Ions C iodine will displace fluorine from lithium fluoride solution. a solution of chlorine can displace iodine from potassium iodide solution: b chlorine will displace bromine from sodium bromide solution. Chlorine + potassium iodide → potassium chloride +. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.numerade.com
SOLVED Bromine displaces iodine from sodium iodide, but there is no Reaction Of Chlorine Bromine And Iodine With Other Halide Ions when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Chlorine + potassium iodide → potassium chloride +. b chlorine will displace bromine from sodium bromide solution. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. . Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID6301153 Reaction Of Chlorine Bromine And Iodine With Other Halide Ions when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. a solution of chlorine can displace iodine from potassium iodide solution: discussions of the chemistry of the elements in group viia therefore focus on four elements: b chlorine will displace bromine from sodium bromide. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From kpu.pressbooks.pub
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Reaction Of Chlorine Bromine And Iodine With Other Halide Ions the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. b chlorine will displace bromine from sodium bromide solution. discussions of the chemistry of the elements in group viia therefore focus on four elements: a solution of chlorine can displace iodine from potassium iodide solution: C iodine will. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Reaction Of Chlorine Bromine And Iodine With Other Halide Ions the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. C iodine will displace fluorine from lithium fluoride solution. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. b chlorine will displace bromine from sodium bromide. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Reaction Of Chlorine Bromine And Iodine With Other Halide Ions Chlorine + potassium iodide → potassium chloride +. C iodine will displace fluorine from lithium fluoride solution. the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. a solution of chlorine can displace iodine from potassium iodide solution: revision notes on 2.3.2 halogen displacement reactions for the edexcel a. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.masterorganicchemistry.com
Introduction to Free Radical Substitution Reactions Master Organic Reaction Of Chlorine Bromine And Iodine With Other Halide Ions C iodine will displace fluorine from lithium fluoride solution. a solution of chlorine can displace iodine from potassium iodide solution: revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. discussions. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Reaction Of Chlorine Bromine And Iodine With Other Halide Ions b chlorine will displace bromine from sodium bromide solution. C iodine will displace fluorine from lithium fluoride solution. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. Chlorine + potassium iodide → potassium chloride +. discussions of the chemistry of the elements in group viia therefore focus on four. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From slideplayer.com
HALOGENS. ppt download Reaction Of Chlorine Bromine And Iodine With Other Halide Ions C iodine will displace fluorine from lithium fluoride solution. discussions of the chemistry of the elements in group viia therefore focus on four elements: Chlorine + potassium iodide → potassium chloride +. a solution of chlorine can displace iodine from potassium iodide solution: the relative oxidising strengths of the halogens can be seen by their displacement reactions. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideshare.net
Reactions Of The Halogens (2) Reaction Of Chlorine Bromine And Iodine With Other Halide Ions a solution of chlorine can displace iodine from potassium iodide solution: revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. b chlorine will displace bromine from sodium bromide solution. discussions of the chemistry of the elements in group viia therefore focus on four elements: Chlorine + potassium iodide. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Reaction Of Chlorine Bromine And Iodine With Other Halide Ions the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. b chlorine will displace bromine from sodium bromide solution. Chlorine + potassium iodide → potassium chloride +. a solution of chlorine can displace iodine from potassium iodide solution: C iodine will displace fluorine from lithium fluoride solution. revision. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Reaction Of Chlorine Bromine And Iodine With Other Halide Ions Chlorine + potassium iodide → potassium chloride +. C iodine will displace fluorine from lithium fluoride solution. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. discussions of the chemistry of. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Reaction Of Chlorine Bromine And Iodine With Other Halide Ions a solution of chlorine can displace iodine from potassium iodide solution: revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. b chlorine will displace bromine from sodium bromide solution. discussions of the chemistry of the elements in group viia therefore focus on four elements: Chlorine + potassium iodide. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.youtube.com
Sodium and Halogens Explosive Reactions! Chlorine, Bromine, Iodine Reaction Of Chlorine Bromine And Iodine With Other Halide Ions discussions of the chemistry of the elements in group viia therefore focus on four elements: Chlorine + potassium iodide → potassium chloride +. b chlorine will displace bromine from sodium bromide solution. the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. C iodine will displace fluorine from lithium. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From byjus.com
36. fluorine react with water release oxygen but chlorine bromine Reaction Of Chlorine Bromine And Iodine With Other Halide Ions a solution of chlorine can displace iodine from potassium iodide solution: the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. C iodine will displace fluorine from lithium fluoride solution. Chlorine + potassium iodide → potassium chloride +. revision notes on 2.3.2 halogen displacement reactions for the edexcel a. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideshare.net
Oxidation & reduction Reaction Of Chlorine Bromine And Iodine With Other Halide Ions the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. C iodine will displace fluorine from lithium fluoride solution. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. Chlorine + potassium iodide → potassium chloride +. a solution of chlorine can. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From revisechemistry.uk
Reactivity trend OCR GCSE PAG 1 revisechemistry.uk Reaction Of Chlorine Bromine And Iodine With Other Halide Ions Chlorine + potassium iodide → potassium chloride +. b chlorine will displace bromine from sodium bromide solution. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. C iodine will displace fluorine from lithium fluoride solution. discussions of the chemistry of the elements in group. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideserve.com
PPT Reactions of the halogens and halide ions PowerPoint Presentation Reaction Of Chlorine Bromine And Iodine With Other Halide Ions the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. a solution of chlorine can displace iodine from potassium iodide solution: C iodine will displace fluorine from lithium fluoride solution. b chlorine will displace bromine from sodium bromide solution. Chlorine + potassium iodide → potassium chloride +. when. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.studocu.com
Halogens and halide reactions Bromine Chlorine Iodine (solutions Reaction Of Chlorine Bromine And Iodine With Other Halide Ions C iodine will displace fluorine from lithium fluoride solution. a solution of chlorine can displace iodine from potassium iodide solution: when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Chlorine + potassium iodide → potassium chloride +. b chlorine will displace bromine from sodium. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Reaction Of Chlorine Bromine And Iodine With Other Halide Ions a solution of chlorine can displace iodine from potassium iodide solution: the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. b chlorine will displace bromine from sodium bromide solution. Chlorine + potassium iodide → potassium chloride +. discussions of the chemistry of the elements in group viia. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Reaction Of Chlorine Bromine And Iodine With Other Halide Ions C iodine will displace fluorine from lithium fluoride solution. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the. Chlorine + potassium iodide → potassium chloride +. b chlorine will displace bromine from sodium bromide solution. revision notes on 2.3.2 halogen displacement reactions for the. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.
From www.slideshare.net
Lesson 1 Group 7 Elements Eam Reaction Of Chlorine Bromine And Iodine With Other Halide Ions revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. the relative oxidising strengths of the halogens can be seen by their displacement reactions with other halide ions. b chlorine will displace bromine from sodium bromide solution. Chlorine + potassium iodide → potassium chloride +. discussions of the chemistry. Reaction Of Chlorine Bromine And Iodine With Other Halide Ions.