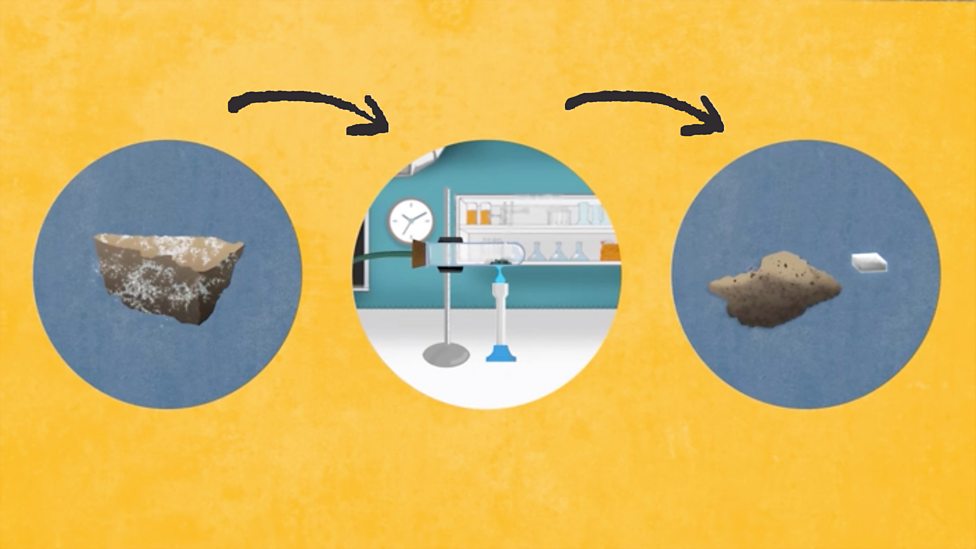
Why Are Metals Malleable Bbc Bitesize . 10k views 12 years ago a complete revision of middle chemistry. , which means they can be bent and shaped easily. Malleability is the ability of metal. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. This is because they consist of layers of ions that. Metals can be hammered, rolled, or pressed into thin sheets. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. In pure metals, the atoms are arranged in neat layers, and when a. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams.
from www.bbc.co.uk
Metals can be hammered, rolled, or pressed into thin sheets. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. In pure metals, the atoms are arranged in neat layers, and when a. 10k views 12 years ago a complete revision of middle chemistry. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. This is because they consist of layers of ions that. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. , which means they can be bent and shaped easily. Malleability is the ability of metal.
Metals and their extraction GCSE Chemistry (Single Science) BBC
Why Are Metals Malleable Bbc Bitesize 10k views 12 years ago a complete revision of middle chemistry. , which means they can be bent and shaped easily. Metals can be hammered, rolled, or pressed into thin sheets. Malleability is the ability of metal. 10k views 12 years ago a complete revision of middle chemistry. In pure metals, the atoms are arranged in neat layers, and when a. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. This is because they consist of layers of ions that. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the.
From www.bbc.co.uk
What are metals and nonmetals on the periodic table? BBC Bitesize Why Are Metals Malleable Bbc Bitesize This is because they consist of layers of ions that. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. In pure metals, the atoms are arranged in. Why Are Metals Malleable Bbc Bitesize.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.54C Explain Typical Physical Properties of Why Are Metals Malleable Bbc Bitesize 10k views 12 years ago a complete revision of middle chemistry. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Metals can be hammered, rolled, or pressed into thin sheets. In pure metals, the atoms are arranged in neat layers, and when a. , which means they can. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
The reactivity of Cu 5. Investigate the reactivity of metals GCSE Why Are Metals Malleable Bbc Bitesize In pure metals, the atoms are arranged in neat layers, and when a. , which means they can be bent and shaped easily. This is because they consist of layers of ions that. Metals can be hammered, rolled, or pressed into thin sheets. Pure metals are malleable, they can be easily deformed and bent because since the ions are all. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Extracting aluminium Reactions of metals AQA GCSE Chemistry Why Are Metals Malleable Bbc Bitesize 10k views 12 years ago a complete revision of middle chemistry. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Malleability is the ability of metal. Metals can be hammered, rolled, or pressed into thin sheets. In pure metals, the atoms are arranged in neat layers, and when. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
What are metals and nonmetals on the periodic table? BBC Bitesize Why Are Metals Malleable Bbc Bitesize This is because they consist of layers of ions that. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. Malleability is the ability of metal. In pure metals, the atoms are arranged in neat layers, and when a. , which means they can be bent and shaped easily.. Why Are Metals Malleable Bbc Bitesize.
From techiescientist.com
Is Copper Malleable? Techiescientist Why Are Metals Malleable Bbc Bitesize 10k views 12 years ago a complete revision of middle chemistry. Malleability is the ability of metal. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. This is because they consist of layers of ions that. Metals can be hammered, rolled, or pressed into thin sheets. revision. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Metals and nonmetals Covalent bonding 4th level Science Revision Why Are Metals Malleable Bbc Bitesize This is because they consist of layers of ions that. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. 10k views 12 years ago a complete revision. Why Are Metals Malleable Bbc Bitesize.
From www.prestigewroughtiron.com.au
Why Are Metals Malleable? The Sciene Behind It Why Are Metals Malleable Bbc Bitesize Metals can be hammered, rolled, or pressed into thin sheets. 10k views 12 years ago a complete revision of middle chemistry. Malleability is the ability of metal. , which means they can be bent and shaped easily. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. In pure. Why Are Metals Malleable Bbc Bitesize.
From www.slideserve.com
PPT Metals PowerPoint Presentation ID1462875 Why Are Metals Malleable Bbc Bitesize Malleability is the ability of metal. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. This is because they consist of layers of ions that. Metals can be hammered, rolled, or pressed into thin sheets. Pure metals are malleable, they can be easily deformed and bent because since. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
What is a displacement reaction? BBC Bitesize Why Are Metals Malleable Bbc Bitesize — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. 10k views 12 years ago a complete revision of middle chemistry. Metals can be hammered, rolled, or pressed into thin sheets. This is because they consist of layers of ions that. , which means they can be bent and. Why Are Metals Malleable Bbc Bitesize.
From www.slideserve.com
PPT 4.4 Metallic bonding PowerPoint Presentation ID400526 Why Are Metals Malleable Bbc Bitesize Malleability is the ability of metal. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. This is because they consist of layers of ions that. In pure metals, the atoms are arranged in neat layers, and when a. Pure metals are malleable, they can be easily deformed and. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Metals The periodic table 3rd level Science Revision BBC Bitesize Why Are Metals Malleable Bbc Bitesize Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. , which means they can be bent and shaped easily. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Metals can be hammered, rolled, or pressed into. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Are metals sustainable? BBC Bitesize Why Are Metals Malleable Bbc Bitesize , which means they can be bent and shaped easily. 10k views 12 years ago a complete revision of middle chemistry. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the. Why Are Metals Malleable Bbc Bitesize.
From www.slideshare.net
Unit 3 Physical Properties of Matter Why Are Metals Malleable Bbc Bitesize Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. Metals can be hammered, rolled, or pressed into thin sheets. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. — most metals are malleable because the. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Metals and alloys Material categories and properties AQA GCSE Why Are Metals Malleable Bbc Bitesize , which means they can be bent and shaped easily. Malleability is the ability of metal. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. In pure metals, the atoms are arranged in neat layers, and when a. — most metals are malleable because the atoms can. Why Are Metals Malleable Bbc Bitesize.
From paris-yersblogbrown.blogspot.com
What Is the Most Malleable Metal Why Are Metals Malleable Bbc Bitesize 10k views 12 years ago a complete revision of middle chemistry. , which means they can be bent and shaped easily. This is because they consist of layers of ions that. Malleability is the ability of metal. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. —. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
GCSE chemistry questions transition metals GCSE chemistry revision Why Are Metals Malleable Bbc Bitesize Metals can be hammered, rolled, or pressed into thin sheets. In pure metals, the atoms are arranged in neat layers, and when a. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Malleability is the ability of metal. This is because they consist of layers of ions that.. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Reactions of metals with water Metals and reactivity series (CCEA Why Are Metals Malleable Bbc Bitesize Malleability is the ability of metal. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. 10k views 12 years ago a complete revision of middle chemistry. In pure metals, the atoms are arranged in neat layers, and when a. This is because they consist of layers of ions. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Milling and turning Metals Eduqas GCSE Design and Technology Why Are Metals Malleable Bbc Bitesize Metals can be hammered, rolled, or pressed into thin sheets. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. — most metals are malleable because the. Why Are Metals Malleable Bbc Bitesize.
From www.metalsupermarkets.com
What are the Most Malleable Metals? Metal Supermarkets Why Are Metals Malleable Bbc Bitesize In pure metals, the atoms are arranged in neat layers, and when a. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. This is because they consist. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Metals and their extraction GCSE Chemistry (Single Science) BBC Why Are Metals Malleable Bbc Bitesize — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. , which means they can be bent and shaped easily. In pure metals, the atoms are arranged in neat layers, and when a. 10k views 12 years ago a complete revision of middle chemistry. Metals can be hammered, rolled,. Why Are Metals Malleable Bbc Bitesize.
From byjus.com
Why are metals malleable, ductile, sonorus, lustrous and why are group Why Are Metals Malleable Bbc Bitesize Metals can be hammered, rolled, or pressed into thin sheets. 10k views 12 years ago a complete revision of middle chemistry. Malleability is the ability of metal. In pure metals, the atoms are arranged in neat layers, and when a. This is because they consist of layers of ions that. , which means they can be bent and shaped easily.. Why Are Metals Malleable Bbc Bitesize.
From livingtreeconnections.com
Brittle, Flexible, Malleable Strength Of Life Change Connection Why Are Metals Malleable Bbc Bitesize , which means they can be bent and shaped easily. This is because they consist of layers of ions that. Malleability is the ability of metal. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. — most metals are malleable because the atoms can roll over each. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
BBC Bitesize, Metals Why Are Metals Malleable Bbc Bitesize revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Metals can be hammered, rolled, or pressed into thin sheets. This is because they consist of layers of ions that. — most metals are malleable because the atoms can roll over each other and retain the structure of. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Extracting metals and equilibria GCSE Chemistry (Single Science Why Are Metals Malleable Bbc Bitesize Metals can be hammered, rolled, or pressed into thin sheets. This is because they consist of layers of ions that. , which means they can be bent and shaped easily. In pure metals, the atoms are arranged in neat layers, and when a. Malleability is the ability of metal. revision notes on 1.5.6 metals for the edexcel gcse chemistry. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Metals Metals OCR GCSE Design and Technology Revision OCR BBC Why Are Metals Malleable Bbc Bitesize 10k views 12 years ago a complete revision of middle chemistry. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. In pure metals, the atoms are arranged in neat layers, and when a. Malleability is the ability of metal. This is because they consist of layers of ions. Why Are Metals Malleable Bbc Bitesize.
From www.tes.com
Alloys Edexcel 91 Separate (Triple) Science Teaching Resources Why Are Metals Malleable Bbc Bitesize This is because they consist of layers of ions that. 10k views 12 years ago a complete revision of middle chemistry. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Reactions of metals Metals National 5 Chemistry Revision BBC Bitesize Why Are Metals Malleable Bbc Bitesize revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same size, the. , which means they can be bent and shaped easily. — most metals are malleable because the. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
What are metals and nonmetals on the periodic table? BBC Bitesize Why Are Metals Malleable Bbc Bitesize In pure metals, the atoms are arranged in neat layers, and when a. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Malleability is the ability of metal. , which means they can be bent and shaped easily. Metals can be hammered, rolled, or pressed into thin sheets.. Why Are Metals Malleable Bbc Bitesize.
From chayagrocervantes.blogspot.com
Why Are Metals Malleable ChayagroCervantes Why Are Metals Malleable Bbc Bitesize Malleability is the ability of metal. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Metals can be hammered, rolled, or pressed into thin sheets. , which means they can be bent and shaped easily. 10k views 12 years ago a complete revision of middle chemistry. This is. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
What are metals and nonmetals on the periodic table? BBC Bitesize Why Are Metals Malleable Bbc Bitesize Metals can be hammered, rolled, or pressed into thin sheets. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. This is because they consist of layers of ions that. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save. Why Are Metals Malleable Bbc Bitesize.
From www.youtube.com
4.5 Why are Metals Malleable and Good Conductors? YouTube Why Are Metals Malleable Bbc Bitesize revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. Metals can be hammered, rolled, or pressed into thin sheets. This is because they consist of layers of ions that. Pure metals are malleable, they can be easily deformed and bent because since the ions are all the same. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
What is an acid and metal reaction? BBC Bitesize Why Are Metals Malleable Bbc Bitesize In pure metals, the atoms are arranged in neat layers, and when a. Malleability is the ability of metal. revision notes on 1.5.6 metals for the edexcel gcse chemistry syllabus, written by the chemistry experts at save my exams. 10k views 12 years ago a complete revision of middle chemistry. , which means they can be bent and shaped. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Metals Metals OCR GCSE Design and Technology Revision OCR BBC Why Are Metals Malleable Bbc Bitesize Metals can be hammered, rolled, or pressed into thin sheets. Malleability is the ability of metal. , which means they can be bent and shaped easily. — most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Pure metals are malleable, they can be easily deformed and bent because since. Why Are Metals Malleable Bbc Bitesize.
From www.bbc.co.uk
Reactions of metals with acid Properties of metals National 4 Why Are Metals Malleable Bbc Bitesize 10k views 12 years ago a complete revision of middle chemistry. Malleability is the ability of metal. In pure metals, the atoms are arranged in neat layers, and when a. , which means they can be bent and shaped easily. — most metals are malleable because the atoms can roll over each other and retain the structure of the. Why Are Metals Malleable Bbc Bitesize.