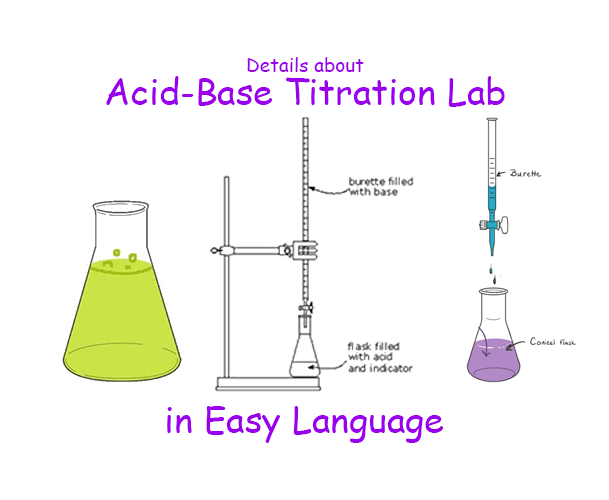
Titration Procedure Use . A burette, calibrated to dispense the titrant, is accurately filled. Titration is a chemical process used to determine the concentration of a substance in a solution. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In all cases, the reaction chosen for the analysis must. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. A chemical reaction is set up between a known volume of a solution of.
from mavink.com
A burette, calibrated to dispense the titrant, is accurately filled. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical reaction is set up between a known volume of a solution of. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Titration is a chemical process used to determine the concentration of a substance in a solution. In all cases, the reaction chosen for the analysis must. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration.
Acid Base Titration Lab Procedure
Titration Procedure Use In all cases, the reaction chosen for the analysis must. In all cases, the reaction chosen for the analysis must. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A burette, calibrated to dispense the titrant, is accurately filled. A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A chemical reaction is set up between a known volume of a solution of. Titration is a chemical process used to determine the concentration of a substance in a solution. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as.
From mungfali.com
Acid Base Titration Procedure Titration Procedure Use In all cases, the reaction chosen for the analysis must. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. A titration is a laboratory technique. Titration Procedure Use.
From www.slideserve.com
PPT ENZYME CATALYSIS LAB PowerPoint Presentation ID5750147 Titration Procedure Use Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a chemical process used to determine the concentration of a substance in a solution. In all cases, the reaction chosen for the analysis must. In this tutorial, you will find information on titration, including. Titration Procedure Use.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Procedure Use Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A chemical reaction is set up between a known volume of a solution of. In all cases, the reaction chosen for the analysis must. Titration is a chemical process used to determine the concentration of a. Titration Procedure Use.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Titration Procedure Use Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. A burette, calibrated to dispense the titrant, is accurately filled. Titration is a chemical process used to determine the concentration of a substance. Titration Procedure Use.
From mavink.com
Titration Procedure Titration Procedure Use Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A burette, calibrated to dispense the titrant, is accurately filled. A titration is a laboratory technique used. Titration Procedure Use.
From www.youtube.com
Titration procedure (Step by step) YouTube Titration Procedure Use Titration is a chemical process used to determine the concentration of a substance in a solution. A chemical reaction is set up between a known volume of a solution of. A burette, calibrated to dispense the titrant, is accurately filled. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions. Titration Procedure Use.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Procedure Use Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. A chemical reaction is set up between a known volume of a solution of. A titration is a laboratory technique used to precisely. Titration Procedure Use.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Procedure Use A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. In all cases, the reaction chosen for the analysis must. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this tutorial, you will find information on titration, including the chemicals. Titration Procedure Use.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Presentation ID1995345 Titration Procedure Use A burette, calibrated to dispense the titrant, is accurately filled. In all cases, the reaction chosen for the analysis must. A chemical reaction is set up between a known volume of a solution of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A precisely measured volume of the. Titration Procedure Use.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Procedure Use Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration Procedure Use.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Procedure Use A burette, calibrated to dispense the titrant, is accurately filled. Titration is a chemical process used to determine the concentration of a substance in a solution. A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. A chemical reaction is set up between a known volume of a solution of. In this. Titration Procedure Use.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Procedure Use A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. A chemical reaction is set up between a known volume of a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A burette, calibrated to. Titration Procedure Use.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Procedure Use In all cases, the reaction chosen for the analysis must. A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. A burette, calibrated to dispense the titrant, is accurately filled. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is. Titration Procedure Use.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Procedure Use In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. Titration Procedure Use.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download ID5498187 Titration Procedure Use Titration is a chemical process used to determine the concentration of a substance in a solution. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical reaction is set up between a known volume of a solution of. A precisely measured volume of the analyte is placed in a. Titration Procedure Use.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Procedure Use A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. Titration is a chemical process used to determine the concentration of a substance in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a procedure used in. Titration Procedure Use.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Procedure Use Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In all cases, the reaction chosen for the analysis must. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. A. Titration Procedure Use.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Procedure Use Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Titration is the slow addition of one solution of a known concentration (called a titrant) to. Titration Procedure Use.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Procedure Use In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. In all cases, the reaction chosen for the analysis must. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a chemical process. Titration Procedure Use.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration TutorMyself Chemistry Titration Procedure Use A burette, calibrated to dispense the titrant, is accurately filled. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. A chemical reaction is set up between a. Titration Procedure Use.
From www.science-revision.co.uk
Titrations Titration Procedure Use In all cases, the reaction chosen for the analysis must. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Titration is the slow addition of. Titration Procedure Use.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base (NaOH) YouTube Titration Procedure Use In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. In all cases, the reaction chosen for the analysis must. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A. Titration Procedure Use.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Procedure Use In all cases, the reaction chosen for the analysis must. A burette, calibrated to dispense the titrant, is accurately filled. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this tutorial, you will find information on titration, including the chemicals that are commonly used. Titration Procedure Use.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Procedure Use A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. Titration is a chemical process used to determine the concentration of a substance in a solution. A chemical reaction is set up between a known volume of a solution of. A titration is a laboratory technique used to precisely measure molar concentration. Titration Procedure Use.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download ID5498187 Titration Procedure Use In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A burette, calibrated to dispense the titrant, is accurately filled. In all cases, the reaction. Titration Procedure Use.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Procedure Use Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A burette, calibrated to dispense the titrant, is accurately filled. Titration is a chemical process used to. Titration Procedure Use.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Procedure Use A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A burette, calibrated to dispense the titrant, is accurately filled. Titration is a chemical process used to determine the concentration of a substance in a solution. A chemical reaction is set up between a known volume of a solution of.. Titration Procedure Use.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Procedure Use A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In all cases, the reaction chosen for the analysis must. Titration is a chemical process used to determine the concentration of. Titration Procedure Use.
From mavink.com
Acid Base Titration Lab Procedure Titration Procedure Use Titration is a chemical process used to determine the concentration of a substance in a solution. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A. Titration Procedure Use.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Procedure Use Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. A chemical reaction is set up between a known volume of a solution of. Titration is a procedure. Titration Procedure Use.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer flask. Pipette and Titration Procedure Use Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In all cases, the reaction chosen for the analysis must. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this tutorial, you will find information on titration, including the. Titration Procedure Use.
From mavink.com
Acid Base Titration Lab Procedure Titration Procedure Use A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In all cases, the reaction chosen for the analysis must. A burette, calibrated to dispense the titrant, is. Titration Procedure Use.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download ID5498187 Titration Procedure Use A burette, calibrated to dispense the titrant, is accurately filled. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. A precisely measured volume of. Titration Procedure Use.
From www.studypool.com
SOLUTION Coulometric titration procedure Studypool Titration Procedure Use Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A chemical reaction is set up between a known volume of a solution of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In all. Titration Procedure Use.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Procedure Use A precisely measured volume of the analyte is placed in a flask or beaker to initiate the titration. Titration is a chemical process used to determine the concentration of a substance in a solution. A burette, calibrated to dispense the titrant, is accurately filled. Titration is the slow addition of one solution of a known concentration (called a titrant) to. Titration Procedure Use.