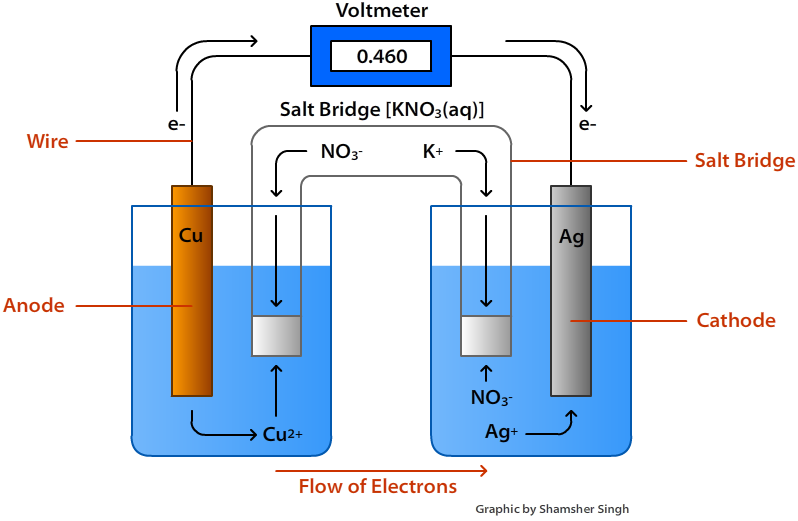
Reaction Of Electrochemical Cell . The half equations can be found using the direction of the electrons. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This flow of charged particles is. Elecrochemical cells with only one metal. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. The electrons flowing from the more reactive element (metal with. What are the half equations?
from chem.libretexts.org
Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. The electrons flowing from the more reactive element (metal with. Elecrochemical cells with only one metal. What are the half equations? The half equations can be found using the direction of the electrons. This flow of charged particles is. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction.
Voltaic Cells Chemistry LibreTexts
Reaction Of Electrochemical Cell The electrons flowing from the more reactive element (metal with. Elecrochemical cells with only one metal. What are the half equations? The half equations can be found using the direction of the electrons. This flow of charged particles is. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. The electrons flowing from the more reactive element (metal with.
From www.youtube.com
9.4.1 Explain how a redox reaction is used to produce electricity in a Reaction Of Electrochemical Cell If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. The half equations can be found using the direction of the electrons. What are the half equations? Elecrochemical cells with only one metal. This flow of charged particles is. Electrochemical cells use redox reactions as the. Reaction Of Electrochemical Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Reaction Of Electrochemical Cell The electrons flowing from the more reactive element (metal with. This flow of charged particles is. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. Elecrochemical cells with only one metal. The half equations can be found using the direction of the electrons. An apparatus. Reaction Of Electrochemical Cell.
From mmerevise.co.uk
Calculating the EMF Reaction Of Electrochemical Cell The electrons flowing from the more reactive element (metal with. What are the half equations? Elecrochemical cells with only one metal. This flow of charged particles is. The half equations can be found using the direction of the electrons. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. If a redox reaction can. Reaction Of Electrochemical Cell.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Reaction Of Electrochemical Cell This flow of charged particles is. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Elecrochemical cells with only one metal. What are the half equations? Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. The half equations. Reaction Of Electrochemical Cell.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID5580086 Reaction Of Electrochemical Cell The half equations can be found using the direction of the electrons. The electrons flowing from the more reactive element (metal with. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Elecrochemical cells with only one metal. What are the half equations? Electrochemical cells use. Reaction Of Electrochemical Cell.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Reaction Of Electrochemical Cell The half equations can be found using the direction of the electrons. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. The electrons flowing from the more reactive element. Reaction Of Electrochemical Cell.
From www.pinterest.com
Electrochemical cell Electrochemistry, Chemistry classroom Reaction Of Electrochemical Cell The electrons flowing from the more reactive element (metal with. The half equations can be found using the direction of the electrons. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. What are the half equations? Electrochemical cells use redox reactions as the electron transfer. Reaction Of Electrochemical Cell.
From www.thoughtco.com
Electrochemical Cell Definition Reaction Of Electrochemical Cell Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. What are the half equations? This flow of charged particles is. The half equations can be found using the direction of the electrons. Elecrochemical cells with only one metal. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely,. Reaction Of Electrochemical Cell.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell Reaction Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Elecrochemical cells with only one metal. What are the half equations? The electrons flowing from the more reactive element (metal. Reaction Of Electrochemical Cell.
From saylordotorg.github.io
Electrochemistry Reaction Of Electrochemical Cell The electrons flowing from the more reactive element (metal with. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. Elecrochemical cells with only one metal. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. What are the half. Reaction Of Electrochemical Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Reaction Of Electrochemical Cell What are the half equations? The electrons flowing from the more reactive element (metal with. Elecrochemical cells with only one metal. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This flow of charged particles is. If a redox reaction can be split into half. Reaction Of Electrochemical Cell.
From flatworldknowledge.lardbucket.org
Electrochemistry Reaction Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The half equations can be found using the direction of the electrons. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. If a redox reaction can be split into. Reaction Of Electrochemical Cell.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of Reaction Of Electrochemical Cell If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. What are the half equations? Elecrochemical cells with only one metal. The half equations can be found using the direction of the electrons. An apparatus that is used to generate electricity from a spontaneous redox reaction. Reaction Of Electrochemical Cell.
From pediaa.com
What is the Difference Between Oxidation and Reduction Electrochemical Reaction Of Electrochemical Cell What are the half equations? The electrons flowing from the more reactive element (metal with. The half equations can be found using the direction of the electrons. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Elecrochemical cells with only one metal. Electrochemical cells use. Reaction Of Electrochemical Cell.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Reaction Of Electrochemical Cell The electrons flowing from the more reactive element (metal with. This flow of charged particles is. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. Elecrochemical cells with only. Reaction Of Electrochemical Cell.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell Reaction Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. What are the half equations? The electrons flowing from the more reactive element (metal with. This flow of charged particles is. Elecrochemical cells with only one metal. The half equations can be found using the direction. Reaction Of Electrochemical Cell.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples Reaction Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This flow of charged particles is. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. The electrons flowing from the more reactive element (metal with. If a redox reaction. Reaction Of Electrochemical Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Reaction Of Electrochemical Cell This flow of charged particles is. Elecrochemical cells with only one metal. The half equations can be found using the direction of the electrons. The electrons flowing from the more reactive element (metal with. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. What are the half equations? An apparatus that is used. Reaction Of Electrochemical Cell.
From studylib.net
Electrochemical cells Reaction Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. The electrons flowing from the more reactive element (metal with. Electrochemical cells. Reaction Of Electrochemical Cell.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Reaction Of Electrochemical Cell Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. What are the half equations? An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Elecrochemical cells with only one metal. This flow of charged particles is. The half equations. Reaction Of Electrochemical Cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Reaction Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. What are the half equations? If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. Electrochemical cells use redox reactions as the. Reaction Of Electrochemical Cell.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 10 simple voltaic cell Reaction Of Electrochemical Cell The half equations can be found using the direction of the electrons. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The electrons flowing from the more reactive element (metal with. This flow of charged particles is. What are the half equations? Electrochemical cells use. Reaction Of Electrochemical Cell.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Reaction Of Electrochemical Cell What are the half equations? This flow of charged particles is. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The electrons flowing from the more reactive element (metal with. If a redox reaction can be split into half reactions it becomes possible to build. Reaction Of Electrochemical Cell.
From www.youtube.com
Redox reaction in an electrolytic cell YouTube Reaction Of Electrochemical Cell If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. This flow of charged particles is. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Elecrochemical cells with only one metal. An apparatus that is used to generate electricity. Reaction Of Electrochemical Cell.
From sites.google.com
Electrochemistry engineering chemistry Reaction Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. Elecrochemical cells with only one metal. What are the half equations? The electrons flowing from the more reactive element (metal. Reaction Of Electrochemical Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Reaction Of Electrochemical Cell Elecrochemical cells with only one metal. This flow of charged particles is. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The half equations can be found using the direction of the electrons. If a redox reaction can be split into half reactions it becomes. Reaction Of Electrochemical Cell.
From enginelistmathew.z13.web.core.windows.net
Cathode In Electrochemical Cell Reaction Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. The half equations can be found using the direction of the electrons.. Reaction Of Electrochemical Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Reaction Of Electrochemical Cell What are the half equations? Elecrochemical cells with only one metal. This flow of charged particles is. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. The half equations can be found using the direction of the electrons. An apparatus that is used to generate. Reaction Of Electrochemical Cell.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Reaction Of Electrochemical Cell If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. What are the half equations? This flow of charged particles is. Elecrochemical cells with only one metal. Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. An apparatus that. Reaction Of Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Reaction Of Electrochemical Cell Elecrochemical cells with only one metal. The half equations can be found using the direction of the electrons. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity. Reaction Of Electrochemical Cell.
From pubs.rsc.org
Making electrochemistry easily accessible to the synthetic chemist Reaction Of Electrochemical Cell Electrochemical cells use redox reactions as the electron transfer between products creates a flow of electrons. The half equations can be found using the direction of the electrons. The electrons flowing from the more reactive element (metal with. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Reaction Of Electrochemical Cell.
From www.pinterest.com
Kognity Chemistry classroom, Electrochemistry, Chemistry lessons Reaction Of Electrochemical Cell What are the half equations? This flow of charged particles is. The half equations can be found using the direction of the electrons. The electrons flowing from the more reactive element (metal with. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. Elecrochemical cells with. Reaction Of Electrochemical Cell.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 11 simple voltaic cell Reaction Of Electrochemical Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This flow of charged particles is. If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. The half equations can be found. Reaction Of Electrochemical Cell.
From www.youtube.com
Emf of daniel cell from nernst equation(Electrochemistry part 28 for Reaction Of Electrochemical Cell This flow of charged particles is. Elecrochemical cells with only one metal. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. The electrons flowing from the more reactive element (metal with. Electrochemical cells use redox reactions as the electron transfer between products creates a flow. Reaction Of Electrochemical Cell.
From wisc.pb.unizin.org
D40.2 Cell Notation Chemistry 109 Fall 2021 Reaction Of Electrochemical Cell If a redox reaction can be split into half reactions it becomes possible to build a device, called an electrochemical cell, that has separate. The half equations can be found using the direction of the electrons. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction.. Reaction Of Electrochemical Cell.