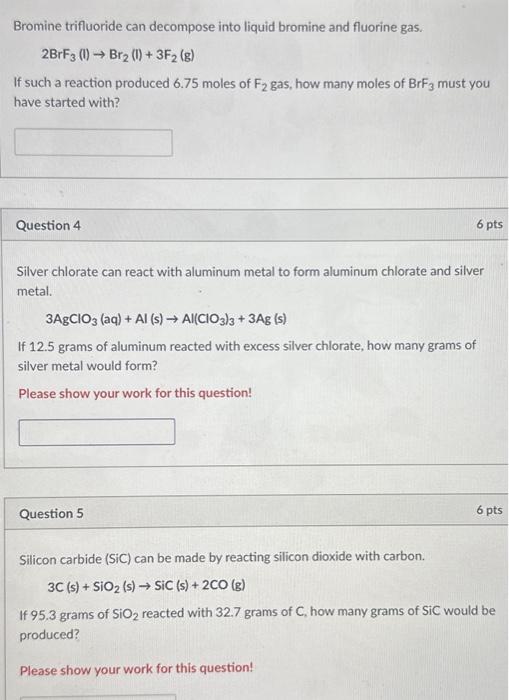
Bromine Trifluoride Fluorine Gas . According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. Bromine trifluoride = dibromine + difluorine. 1 convert mass f2 to mol f2 using the molar mass of f2; 2 convert mol f2 to mol brf3 using the. We want 29.2 g of flourine gas, f 2. Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or.
from www.chegg.com
1 convert mass f2 to mol f2 using the molar mass of f2; What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Bromine trifluoride = dibromine + difluorine. Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? We want 29.2 g of flourine gas, f 2. 2 convert mol f2 to mol brf3 using the.
Solved Please let me know asap!!Bromine trifluoride can
Bromine Trifluoride Fluorine Gas Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. 1 convert mass f2 to mol f2 using the molar mass of f2; According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? We want 29.2 g of flourine gas, f 2. Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Bromine trifluoride = dibromine + difluorine. Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. 2 convert mol f2 to mol brf3 using the.
From wellcometreeoflife.org
BrF3 Molecular Geometry (2021) Everything You Need to Know Bromine Trifluoride Fluorine Gas 1 convert mass f2 to mol f2 using the molar mass of f2; Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. We want 29.2 g of flourine gas, f 2. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572. Bromine Trifluoride Fluorine Gas.
From www.numerade.com
SOLVED a) Calcium metal reacts with water to produce calcium hydroxide Bromine Trifluoride Fluorine Gas Bromine trifluoride = dibromine + difluorine. 1 convert mass f2 to mol f2 using the molar mass of f2; According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? 2 convert mol f2 to mol brf3 using the. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride. Bromine Trifluoride Fluorine Gas.
From www.youtube.com
Is BrF3 (Bromine trifluoride) Ionic or Covalent/Molecular? YouTube Bromine Trifluoride Fluorine Gas We want 29.2 g of flourine gas, f 2. 1 convert mass f2 to mol f2 using the molar mass of f2; Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. 2 convert mol f2 to mol brf3 using the. What volume of fluorine gas is produced. Bromine Trifluoride Fluorine Gas.
From www.chegg.com
Solved Please let me know asap!!Bromine trifluoride can Bromine Trifluoride Fluorine Gas 2 convert mol f2 to mol brf3 using the. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Bromine trifluoride = dibromine + difluorine. Using the molar mass of f. Bromine Trifluoride Fluorine Gas.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Fluorine Gas Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. 1 convert mass f2 to mol f2 using the molar mass of f2; Bromine trifluoride = dibromine + difluorine. 2 convert mol f2 to mol brf3 using the. What volume of fluorine gas is produced when 0.148 mol. Bromine Trifluoride Fluorine Gas.
From techiescientist.com
BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Bromine Trifluoride Fluorine Gas Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. We want 29.2 g of flourine gas, f 2. Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. According to the following reaction, how many moles of bromine trifluoride are. Bromine Trifluoride Fluorine Gas.
From www.dreamstime.com
Bromine fluoride stock illustration. Illustration of molecular 8439119 Bromine Trifluoride Fluorine Gas According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? 2 convert mol f2 to mol brf3 using the. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Brf3 = br2 + f2 is a decomposition reaction where two moles. Bromine Trifluoride Fluorine Gas.
From exobvmtcw.blob.core.windows.net
Bromine Fluoride Structure at Guadalupe Lewis blog Bromine Trifluoride Fluorine Gas We want 29.2 g of flourine gas, f 2. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? 1 convert mass f2 to mol f2 using the molar mass of f2; Bromine trifluoride = dibromine + difluorine. Using the molar mass of f 2 , 38 g/mole, we can calculate. Bromine Trifluoride Fluorine Gas.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Fluorine Gas Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. We want 29.2 g of flourine gas, f 2. 1 convert mass f2 to mol f2 using the molar mass of f2; What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according. Bromine Trifluoride Fluorine Gas.
From imgbin.com
Chlorine Trifluoride Chlorine Pentafluoride Boron Trifluoride Chlorine Bromine Trifluoride Fluorine Gas Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. Bromine trifluoride = dibromine + difluorine. 1 convert mass f2 to mol f2 using the molar mass of f2; According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas?. Bromine Trifluoride Fluorine Gas.
From www.youtube.com
BrF3 Bromine Trifluoride Shape Hybridisation VSEPR Problem Bromine Trifluoride Fluorine Gas Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. 1 convert mass f2 to mol f2 using the molar mass of f2; We want 29.2 g of flourine gas, f 2. Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole),. Bromine Trifluoride Fluorine Gas.
From www.youtube.com
Lewis Structure of BrF3 (bromine trifluoride) YouTube Bromine Trifluoride Fluorine Gas 1 convert mass f2 to mol f2 using the molar mass of f2; We want 29.2 g of flourine gas, f 2. 2 convert mol f2 to mol brf3 using the. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Brf3 = br2 + f2 is a decomposition reaction. Bromine Trifluoride Fluorine Gas.
From www.pinterest.com
BrF3 Polar or Nonpolar? (Bromine Trifluoride) Molecules, Chemical Bromine Trifluoride Fluorine Gas 2 convert mol f2 to mol brf3 using the. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? 1 convert mass f2 to mol f2 using the molar mass of f2; Bromine trifluoride = dibromine + difluorine. We want 29.2 g of flourine gas, f 2. Brf3 = br2 +. Bromine Trifluoride Fluorine Gas.
From www.numerade.com
SOLVED According to the following reaction, how many grams of bromine Bromine Trifluoride Fluorine Gas 1 convert mass f2 to mol f2 using the molar mass of f2; Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. Bromine trifluoride = dibromine + difluorine. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? 2 convert mol f2 to. Bromine Trifluoride Fluorine Gas.
From ar.inspiredpencil.com
Br2 Lewis Structure Bromine Trifluoride Fluorine Gas 1 convert mass f2 to mol f2 using the molar mass of f2; What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. According to the following reaction, how many moles of bromine. Bromine Trifluoride Fluorine Gas.
From www.chemtube3d.com
BrF3 Bromine trifluoride Bromine Trifluoride Fluorine Gas We want 29.2 g of flourine gas, f 2. Bromine trifluoride = dibromine + difluorine. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? Brf3 = br2 + f2 is. Bromine Trifluoride Fluorine Gas.
From www.numerade.com
SOLVED What volume of bromine is produced when 94.4 liters of bromine Bromine Trifluoride Fluorine Gas Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. 1 convert mass f2 to mol f2 using the molar mass of f2; What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. We want 29.2 g of flourine gas, f 2. Bromine. Bromine Trifluoride Fluorine Gas.
From www.bartleby.com
Answered What volume of bromine trifluoride is… bartleby Bromine Trifluoride Fluorine Gas Bromine trifluoride = dibromine + difluorine. 1 convert mass f2 to mol f2 using the molar mass of f2; According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. What volume of fluorine gas. Bromine Trifluoride Fluorine Gas.
From www.smtgases.com
Bromine trifluoride, BrF3, Bromine Trifluoride Fluorine Gas Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. We. Bromine Trifluoride Fluorine Gas.
From www.numerade.com
SOLVED What volume of bromine trifluoride is required to produce 156 Bromine Trifluoride Fluorine Gas Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Bromine. Bromine Trifluoride Fluorine Gas.
From www.youtube.com
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube Bromine Trifluoride Fluorine Gas What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. 1 convert mass f2 to mol f2 using the molar mass of f2; Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. Bromine trifluoride = dibromine + difluorine. Using the molar mass. Bromine Trifluoride Fluorine Gas.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Fluorine Gas Bromine trifluoride = dibromine + difluorine. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? We want 29.2 g of flourine gas, f 2. 1 convert mass f2 to mol. Bromine Trifluoride Fluorine Gas.
From chem.libretexts.org
10.5 Bromine Trifluoride as a Solvent Chemistry LibreTexts Bromine Trifluoride Fluorine Gas Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? 2 convert mol f2 to mol brf3 using the. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely. Bromine Trifluoride Fluorine Gas.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Fluorine Gas 2 convert mol f2 to mol brf3 using the. We want 29.2 g of flourine gas, f 2. 1 convert mass f2 to mol f2 using the molar mass of f2; According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? Using the molar mass of f 2 , 38 g/mole,. Bromine Trifluoride Fluorine Gas.
From in.pinterest.com
Bromine trifluoride has the chemical formula BrF3 and interhalogen Bromine Trifluoride Fluorine Gas 2 convert mol f2 to mol brf3 using the. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. Brf3 = br2 + f2 is a decomposition reaction. Bromine Trifluoride Fluorine Gas.
From www.alamy.com
BrF3 bromine trifluoride CAS 7787715 chemical substance in white Bromine Trifluoride Fluorine Gas Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. 2 convert mol f2 to mol brf3 using the. We want 29.2 g of flourine gas, f 2. According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? 1 convert mass f2 to mol. Bromine Trifluoride Fluorine Gas.
From chem.libretexts.org
10.5 Bromine Trifluoride as a Solvent Chemistry LibreTexts Bromine Trifluoride Fluorine Gas 1 convert mass f2 to mol f2 using the molar mass of f2; According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? We want 29.2 g of flourine gas, f 2. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following. Bromine Trifluoride Fluorine Gas.
From www.dreamstime.com
Bromine fluoride molecule stock illustration. Illustration of bonding Bromine Trifluoride Fluorine Gas 1 convert mass f2 to mol f2 using the molar mass of f2; 2 convert mol f2 to mol brf3 using the. Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole),. Bromine Trifluoride Fluorine Gas.
From favpng.com
Interhalogen Bromine Pentafluoride Chlorine Trifluoride Iodine Bromine Trifluoride Fluorine Gas 1 convert mass f2 to mol f2 using the molar mass of f2; Using the molar mass of f 2 , 38 g/mole, we can calculate this to be (29.2 g /38 g /mole), or. Bromine trifluoride = dibromine + difluorine. We want 29.2 g of flourine gas, f 2. 2 convert mol f2 to mol brf3 using the. Brf3. Bromine Trifluoride Fluorine Gas.
From www.youtube.com
non aqueous solvent. liquid bromine trifluoride YouTube Bromine Trifluoride Fluorine Gas Bromine trifluoride = dibromine + difluorine. We want 29.2 g of flourine gas, f 2. 2 convert mol f2 to mol brf3 using the. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Using the molar mass of f 2 , 38 g/mole, we can calculate this to be. Bromine Trifluoride Fluorine Gas.
From blog.naver.com
브롬 트리플루오르화합물(BROMINE TRIFLUORIDE, BrF3) 네이버 블로그 Bromine Trifluoride Fluorine Gas 1 convert mass f2 to mol f2 using the molar mass of f2; What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. According to the following reaction, how many moles of bromine. Bromine Trifluoride Fluorine Gas.
From www.numerade.com
SOLVED a) Calcium metal reacts with water to produce calcium hydroxide Bromine Trifluoride Fluorine Gas We want 29.2 g of flourine gas, f 2. Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. 2 convert mol f2 to mol brf3 using the. Bromine trifluoride = dibromine + difluorine. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following. Bromine Trifluoride Fluorine Gas.
From www.numerade.com
SOLVED Draw the Lewis dot structures of the chemicals below sulfate Bromine Trifluoride Fluorine Gas Bromine trifluoride = dibromine + difluorine. 1 convert mass f2 to mol f2 using the molar mass of f2; According to the following reaction, how many moles of bromine trifluoride are necessary to form 0.572 moles fluorine gas? We want 29.2 g of flourine gas, f 2. Brf3 = br2 + f2 is a decomposition reaction where two moles of. Bromine Trifluoride Fluorine Gas.
From ar.inspiredpencil.com
Bromine Trifluoride Bromine Trifluoride Fluorine Gas Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. 2 convert mol f2 to mol brf3 using the. 1 convert mass f2 to mol f2 using the molar mass of f2; Using. Bromine Trifluoride Fluorine Gas.
From exoqhrsip.blob.core.windows.net
Bromine Trifluoride(G) Bromine(G) + Fluorine(G) at Darrell Wallace blog Bromine Trifluoride Fluorine Gas Brf3 = br2 + f2 is a decomposition reaction where two moles of bromine trifluoride [brf 3]. 2 convert mol f2 to mol brf3 using the. 1 convert mass f2 to mol f2 using the molar mass of f2; What volume of fluorine gas is produced when 0.148 mol of bromine trifluoride reacts completely according to the following reaction. We. Bromine Trifluoride Fluorine Gas.