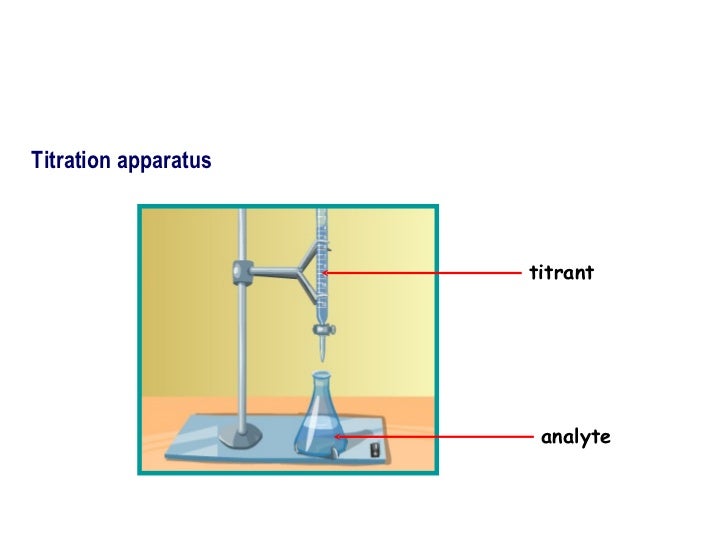
Can An Analyte Be A Titrant . A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. There are two key substances in a titration: The titrant is a solution of known concentration that is used to. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. The analyte (titrand) is the solution with an. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. The titrant and the analyte.
from www.slideshare.net
Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The titrant and the analyte. The titrant is a solution of known concentration that is used to. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. There are two key substances in a titration: The analyte (titrand) is the solution with an.
Acid base titration
Can An Analyte Be A Titrant The titrant and the analyte. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The titrant is a solution of known concentration that is used to. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The titrant and the analyte. The analyte (titrand) is the solution with an. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. There are two key substances in a titration:
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Can An Analyte Be A Titrant The analyte (titrand) is the solution with an. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Can An Analyte Be A Titrant.
From www.slideserve.com
PPT Titrimetry PowerPoint Presentation, free download ID2261229 Can An Analyte Be A Titrant Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The analyte (titrand) is the solution with an. The titrant. Can An Analyte Be A Titrant.
From slideplayer.com
Chapter 7 Let the Titrations Begin ppt download Can An Analyte Be A Titrant There are two key substances in a titration: Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Can An Analyte Be A Titrant.
From studyespacioec4o.z22.web.core.windows.net
How To Do Titrations In Chemistry Can An Analyte Be A Titrant The titrant is a solution of known concentration that is used to. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Can An Analyte Be A Titrant.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Can An Analyte Be A Titrant There are two key substances in a titration: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Can An Analyte Be A Titrant.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Can An Analyte Be A Titrant Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. The analyte (titrand) is the solution with an. The titrant is a solution of known concentration that is used to. There are two key substances in a titration: In an indicator based titration you add another chemical. Can An Analyte Be A Titrant.
From www.slideserve.com
PPT Titrimetry PowerPoint Presentation, free download ID8868517 Can An Analyte Be A Titrant The titrant and the analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. There are two key substances in a titration: The titrant is a solution of known concentration that is used to. Titration involves the gradual. Can An Analyte Be A Titrant.
From differencebtw.com
Titrant vs. Analyte Know the Difference Can An Analyte Be A Titrant A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The titrant and the analyte. The titrant is a solution of known concentration that is used to. There are two key substances in a titration: The analyte (titrand) is. Can An Analyte Be A Titrant.
From www.aquaportail.com
Analyte définition et explications Can An Analyte Be A Titrant A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. There are two key substances in a titration: Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of. Can An Analyte Be A Titrant.
From www.slideserve.com
PPT INTRODUCTION TO TITRIMETRY PowerPoint Presentation, free download Can An Analyte Be A Titrant Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The titrant is a solution of known concentration that is. Can An Analyte Be A Titrant.
From chem.libretexts.org
Quantitative Analysis Nuts and Bolts Chemistry LibreTexts Can An Analyte Be A Titrant The analyte (titrand) is the solution with an. The titrant is a solution of known concentration that is used to. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a volumetric technique in which a solution of one reactant. Can An Analyte Be A Titrant.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Can An Analyte Be A Titrant There are two key substances in a titration: The titrant is a solution of known concentration that is used to. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. The analyte (titrand) is the solution with an. Titration involves the gradual addition of a reagent of. Can An Analyte Be A Titrant.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Can An Analyte Be A Titrant In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The titrant and the analyte. The. Can An Analyte Be A Titrant.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 Can An Analyte Be A Titrant The titrant is a solution of known concentration that is used to. The analyte (titrand) is the solution with an. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is an analytical technique that allows the quantitative determination of a specific. Can An Analyte Be A Titrant.
From www.slideserve.com
PPT Titrimetry PowerPoint Presentation, free download ID2261229 Can An Analyte Be A Titrant A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. The titrant and the analyte. The. Can An Analyte Be A Titrant.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Can An Analyte Be A Titrant Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. The analyte (titrand) is the solution with an. There are. Can An Analyte Be A Titrant.
From slideplayer.com
Types of Chemical Reactions and Solution Stoichiometry ppt download Can An Analyte Be A Titrant Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The titrant is a solution of known concentration that is used to. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Can An Analyte Be A Titrant.
From slideplayer.com
Types of Chemical Reactions & Solution Stoichiometry ppt download Can An Analyte Be A Titrant The titrant and the analyte. The titrant is a solution of known concentration that is used to. There are two key substances in a titration: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is an analytical technique that allows the. Can An Analyte Be A Titrant.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Can An Analyte Be A Titrant Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. There are two key substances in a titration: In an. Can An Analyte Be A Titrant.
From www.askdifference.com
Titrant vs. Analyte — What’s the Difference? Can An Analyte Be A Titrant Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The titrant is a solution of known concentration that is used to. The titrant and the analyte. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in. Can An Analyte Be A Titrant.
From slideplayer.com
Conservation of Atoms Main Concept ppt download Can An Analyte Be A Titrant A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The analyte (titrand) is the solution with an. The titrant is a solution of known concentration that is used to. Titration is an analytical technique that allows the quantitative. Can An Analyte Be A Titrant.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Can An Analyte Be A Titrant Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. There are two key substances in. Can An Analyte Be A Titrant.
From www.numerade.com
SOLVED Label the titration setup below and indicate (place an arrow Can An Analyte Be A Titrant A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. The titrant is a solution of. Can An Analyte Be A Titrant.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Can An Analyte Be A Titrant There are two key substances in a titration: The titrant and the analyte. The titrant is a solution of known concentration that is used to. The analyte (titrand) is the solution with an. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Titration involves the gradual. Can An Analyte Be A Titrant.
From www.microlit.com
An Advanced Guide to Titration Microlit Can An Analyte Be A Titrant The analyte (titrand) is the solution with an. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Can An Analyte Be A Titrant.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Can An Analyte Be A Titrant Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The titrant is a solution of known concentration that is. Can An Analyte Be A Titrant.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Can An Analyte Be A Titrant The titrant is a solution of known concentration that is used to. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In an indicator based titration you add another chemical that changes color at the ph equal to. Can An Analyte Be A Titrant.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation ID3254207 Can An Analyte Be A Titrant There are two key substances in a titration: Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. The titrant is a solution of known concentration that is used to. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Can An Analyte Be A Titrant.
From chimactiv.agroparistech.fr
Chimactiv Interactive numerical educational resources for the Can An Analyte Be A Titrant Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The titrant is a solution of known concentration that is. Can An Analyte Be A Titrant.
From www.slideserve.com
PPT CH 103 ACIDBASE TITRATIONS PowerPoint Presentation, free Can An Analyte Be A Titrant A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. The titrant is a solution of. Can An Analyte Be A Titrant.
From www.slideshare.net
Acid base titration Can An Analyte Be A Titrant The titrant and the analyte. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a volumetric. Can An Analyte Be A Titrant.
From www.numerade.com
SOLVED Under basic conditions, MnO4 can be used as a titrant for the Can An Analyte Be A Titrant There are two key substances in a titration: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of. Can An Analyte Be A Titrant.
From www.chegg.com
Solved 1. Objectives 1. To demonstrate the basic laboratory Can An Analyte Be A Titrant In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The titrant is a solution of known concentration that is. Can An Analyte Be A Titrant.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Can An Analyte Be A Titrant The titrant and the analyte. The analyte (titrand) is the solution with an. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid. There are two key substances in a titration: Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in. Can An Analyte Be A Titrant.
From theedge.com.hk
Chemistry How To Titration The Edge Can An Analyte Be A Titrant A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The analyte. Can An Analyte Be A Titrant.