Chlorine Isotope Ms . There are two stable isotopes, 35 cl. This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. The atomic weight of chlorine given on the periodic table is 35.47 u. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 35 cl and 37 cl with. Chlorine is an excellent example of how isotope distributions are useful for interpretation. Chlorine has two stable isotopes: There are only two stable isotopes: It also deals briefly with the origin of the m+4 peak. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. Different isotopes have different relative. The molecular weight of chlorine is 35.45 u. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide:
from www.semanticscholar.org
The molecular weight of chlorine is 35.45 u. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. There are only two stable isotopes: This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 35 cl and 37 cl with. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine has two stable isotopes: It also deals briefly with the origin of the m+4 peak. The atomic weight of chlorine given on the periodic table is 35.47 u.
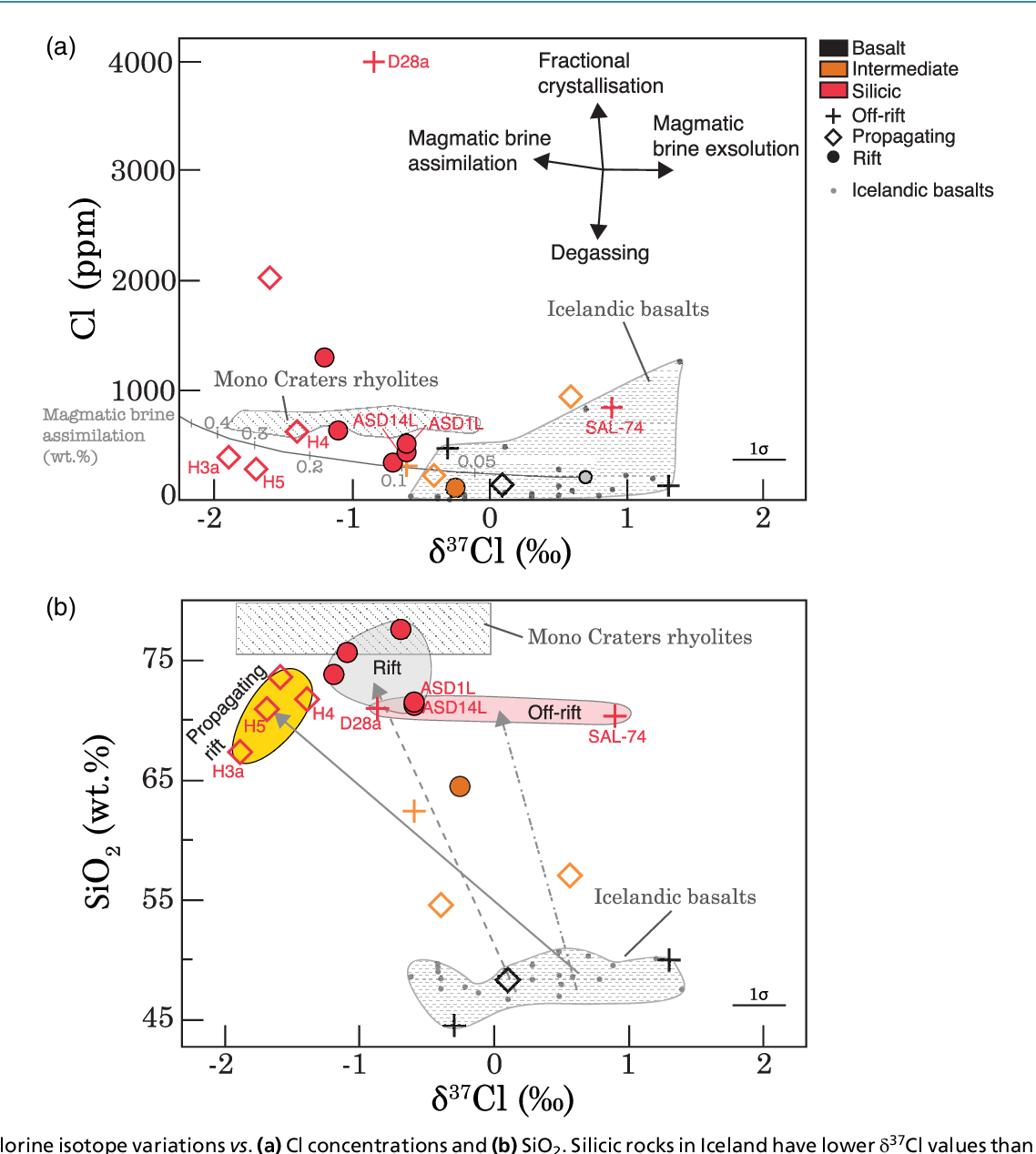
Figure 1 from Chlorine isotope ratios record magmatic brine
Chlorine Isotope Ms It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. Different isotopes have different relative. It also deals briefly with the origin of the m+4 peak. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: Chlorine has two stable isotopes: There are only two stable isotopes: It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 35 cl and 37 cl with. Chlorine is an excellent example of how isotope distributions are useful for interpretation. The molecular weight of chlorine is 35.45 u. The atomic weight of chlorine given on the periodic table is 35.47 u. There are two stable isotopes, 35 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound.
From www.coursehero.com
[Solved] Chlorine has two naturally occurring isotopes Chlorine 35 Chlorine Isotope Ms The atomic weight of chlorine given on the periodic table is 35.47 u. Chlorine is an excellent example of how isotope distributions are useful for interpretation. There are two stable isotopes, 35 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. It shows how you. Chlorine Isotope Ms.
From www.slideshare.net
Atomic Structure Part 2 Chlorine Isotope Ms Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m. Chlorine Isotope Ms.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 Chlorine Isotope Ms Different isotopes have different relative. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The atomic weight of chlorine given on the periodic table is 35.47 u. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic. Chlorine Isotope Ms.
From www.slideserve.com
PPT Isotopes & Average Atomic Mass PowerPoint Presentation, free Chlorine Isotope Ms This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. The molecular weight of chlorine is 35.45 u. Chlorine is an excellent example of how isotope distributions are useful for interpretation. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl,. Chlorine Isotope Ms.
From www.alamy.com
Chlorine chemical element isotopes atomic structure illustration Chlorine Isotope Ms Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. 35 cl and 37 cl with. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. Chlorine is. Chlorine Isotope Ms.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Chlorine Isotope Ms There are only two stable isotopes: Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: Different isotopes have different relative. It also deals briefly with the origin of the m+4 peak. The atomic weight of chlorine given on the periodic table is 35.47 u. Because there are two abundant isotopes of both chlorine. Chlorine Isotope Ms.
From ar.inspiredpencil.com
Chlorine Isotope Chlorine Isotope Ms Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. Different isotopes have different relative. 35 cl and 37 cl with. This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. There are only two stable isotopes: Because there are two abundant. Chlorine Isotope Ms.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Chlorine Isotope Ms 35 cl and 37 cl with. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. There are two stable isotopes, 35 cl. Chlorine is an excellent example of how isotope distributions are useful for interpretation. Chlorine has two stable isotopes: This page explains how the m+2 peak in a mass spectrum arises from the presence. Chlorine Isotope Ms.
From kpu.docbrown.info
C6H5Cl mass spectrum of chlorobenzene fragmentation pattern of m/z m/e Chlorine Isotope Ms Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. The atomic weight of chlorine given on the periodic table is 35.47 u. Different isotopes have different relative. There are only two. Chlorine Isotope Ms.
From scienceprism.co.uk
How archaeologists use chemistry to find out about our past Science Prism Chlorine Isotope Ms It also deals briefly with the origin of the m+4 peak. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: The molecular weight of chlorine is 35.45 u. Chlorine is an excellent example of how isotope distributions are useful for interpretation. This page explains how the m+2 peak in a mass spectrum arises. Chlorine Isotope Ms.
From www.youtube.com
Isotopes of chlorine (Part 21), General Science Class 8 to 12, MTSE Chlorine Isotope Ms Chlorine is an excellent example of how isotope distributions are useful for interpretation. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. 53 rows chlorine (17 cl) has 25 isotopes, ranging. Chlorine Isotope Ms.
From www.alamy.com
Chlorine chemical element isotopes atomic structure illustration Chlorine Isotope Ms There are two stable isotopes, 35 cl. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50%. Chlorine Isotope Ms.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Chlorine Isotope Ms The atomic weight of chlorine given on the periodic table is 35.47 u. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. There are two stable isotopes, 35 cl. The molecular. Chlorine Isotope Ms.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine Isotope Ms Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 35 cl and 37 cl with. There are only two stable isotopes: It also deals briefly with the origin of the m+4 peak. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information. Chlorine Isotope Ms.
From ar.inspiredpencil.com
Chlorine Isotopes Chlorine Isotope Ms It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are only two stable. Chlorine Isotope Ms.
From www.researchgate.net
Stable isotopes of chlorine Download Scientific Diagram Chlorine Isotope Ms Chlorine is an excellent example of how isotope distributions are useful for interpretation. It also deals briefly with the origin of the m+4 peak. Chlorine has two stable isotopes: This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. The atomic weight of chlorine given on. Chlorine Isotope Ms.
From pixels.com
Isotopes Of Chlorine Photograph by Photo Libary Chlorine Isotope Ms Chlorine has two stable isotopes: This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. Different isotopes have different relative. It also deals briefly with the origin of the m+4 peak. Chlorine is an excellent example of how isotope distributions are useful for interpretation. 35 cl. Chlorine Isotope Ms.
From www.sepscience.com
The Role of Isotope Peak Intensities Obtained Using Mass Spectrometry Chlorine Isotope Ms This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 35 cl and 37 cl with. Different isotopes have different relative. There are only two stable isotopes: There are two stable isotopes,. Chlorine Isotope Ms.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Chlorine Isotope Ms The molecular weight of chlorine is 35.45 u. It also deals briefly with the origin of the m+4 peak. Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. There are two. Chlorine Isotope Ms.
From www.semanticscholar.org
Figure 1 from Chlorine isotope ratios record magmatic brine Chlorine Isotope Ms Chlorine has two stable isotopes: 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element.. Chlorine Isotope Ms.
From www.researchgate.net
B represents the part of the spectrum containing the isotope pattern of Chlorine Isotope Ms Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The atomic weight of chlorine given on the periodic table is 35.47 u. It also deals briefly with the origin of the m+4 peak. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m. Chlorine Isotope Ms.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Isotope Ms Chlorine has two stable isotopes: Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. This page explains how the m+2 peak. Chlorine Isotope Ms.
From ar.inspiredpencil.com
Isotopes Of Chlorine Chlorine Isotope Ms Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: Because there are two abundant isotopes of both chlorine (about 75% 35 cl and 25% 37 cl) and bromine (about 50% 79 br and 50% 81 br), chlorinated and brominated compounds have very large and recognizable m+2 peaks. This page explains how the m+2. Chlorine Isotope Ms.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Chlorine Isotope Ms 35 cl and 37 cl with. Chlorine has two stable isotopes: It also deals briefly with the origin of the m+4 peak. Different isotopes have different relative. This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. Because there are two abundant isotopes of both chlorine. Chlorine Isotope Ms.
From byjus.com
cl 35 and cl 37 are two isotopes of chlorine . if average atomic mass Chlorine Isotope Ms Different isotopes have different relative. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl.. Chlorine Isotope Ms.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6463 Science Chlorine Isotope Ms Chlorine has two stable isotopes: There are only two stable isotopes: Chlorine is an excellent example of how isotope distributions are useful for interpretation. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: The atomic weight of chlorine given on the periodic table is 35.47 u. Because there are two abundant isotopes of. Chlorine Isotope Ms.
From www.science-revision.co.uk
Isotopes Chlorine Isotope Ms 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. Chlorine is an excellent example. Chlorine Isotope Ms.
From slideplayer.com
Advanced Pharmaceutical Analysis Mass spectrometry ppt download Chlorine Isotope Ms There are two stable isotopes, 35 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine is an excellent example of how isotope distributions are useful for interpretation. This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine. Chlorine Isotope Ms.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine Isotope Ms There are only two stable isotopes: Chlorine is an excellent example of how isotope distributions are useful for interpretation. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: The molecular weight of chlorine is 35.45 u. 35 cl and 37 cl with. Chlorine has 24 isotopes with mass numbers ranging from 28 cl. Chlorine Isotope Ms.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine Isotope Ms 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The molecular weight of chlorine is 35.45 u. There are two stable isotopes, 35 cl. There are only two stable isotopes: Chlorine has two stable isotopes: Because there are two abundant isotopes of both chlorine (about 75%. Chlorine Isotope Ms.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Isotope Ms Chlorine has two stable isotopes: 35 cl and 37 cl with. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: There are two stable isotopes, 35 cl. Chlorine is an excellent example of how isotope distributions are useful for. Chlorine Isotope Ms.
From ar.inspiredpencil.com
Isotopes Of Chlorine Chlorine Isotope Ms 35 cl and 37 cl with. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. It also deals briefly with the origin of the m+4 peak. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The molecular weight of chlorine is. Chlorine Isotope Ms.
From ar.inspiredpencil.com
Isotopes Of Chlorine Chlorine Isotope Ms It also deals briefly with the origin of the m+4 peak. This page explains how the m+2 peak in a mass spectrum arises from the presence of chlorine or bromine atoms in an organic compound. It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate. Chlorine Isotope Ms.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 Chlorine Isotope Ms Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. Fragments containing both isotopes of br can be seen in the mass spectrum of ethyl bromide: The atomic weight of chlorine given on the periodic table is 35.47 u. Different isotopes have different relative. There are two stable isotopes, 35 cl. 53 rows chlorine (17 cl). Chlorine Isotope Ms.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Chlorine Isotope Ms It shows how you can find out the masses and relative abundances of the various isotopes of the element and use that information to calculate the relative atomic mass of the element. The atomic weight of chlorine given on the periodic table is 35.47 u. There are two stable isotopes, 35 cl. Chlorine is an excellent example of how isotope. Chlorine Isotope Ms.