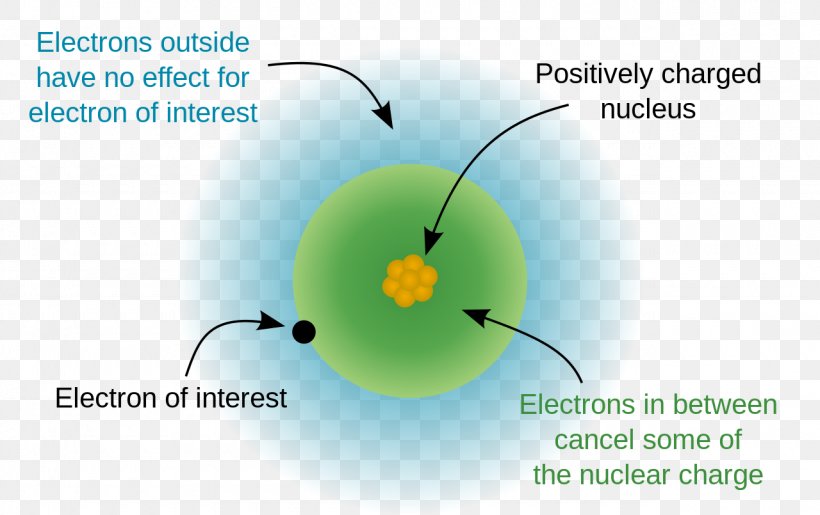
Define Shielding Effect And Effective Nuclear Charge . The shielding effect explains why. The effective nuclear charge may be approximated by the equation:. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,.
from favpng.com
The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The effective nuclear charge may be approximated by the equation:. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The shielding effect explains why.
Effective Nuclear Charge Shielding Effect Atomic Nucleus Atomic Number
Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The shielding effect explains why. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons.
From www.youtube.com
Lesson 9 Effective Nuclear Charge & Shielding Effect Topic Periodic Define Shielding Effect And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The shielding effect explains why. The effective nuclear charge may be approximated by the equation:. The shielding effect describes the balance between the. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Shielding Effect Screening Effect Z eff effective nuclear charge Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The shielding effect explains why. The shielding effect can be defined as a reduction in the effective nuclear charge on the. Define Shielding Effect And Effective Nuclear Charge.
From www.slideserve.com
PPT Chapter 6 PowerPoint Presentation, free download ID4499242 Define Shielding Effect And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a. Define Shielding Effect And Effective Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps Define Shielding Effect And Effective Nuclear Charge The effective nuclear charge may be approximated by the equation:. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Nuclear Charge, Shielding Effect & Effective Nuclear Charge (Singapore Define Shielding Effect And Effective Nuclear Charge The shielding effect explains why. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The effective nuclear charge may be approximated by the equation:. The shielding effect describes the balance between the pull of the protons on valence electrons. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Define Shielding Effect And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The. Define Shielding Effect And Effective Nuclear Charge.
From www.slideserve.com
PPT Chapter 6 Periodic Trends PowerPoint Presentation, free download Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The concept of electron shielding, in which intervening. Define Shielding Effect And Effective Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps Define Shielding Effect And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The shielding effect explains why. The effective nuclear charge may be approximated by the equation:.. Define Shielding Effect And Effective Nuclear Charge.
From gamma.app
Understanding Shielding Effect and Effective Nuclear Charge Define Shielding Effect And Effective Nuclear Charge The shielding effect explains why. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The concept of electron shielding, in which intervening electrons act to reduce the. Define Shielding Effect And Effective Nuclear Charge.
From favpng.com
Effective Nuclear Charge Shielding Effect Atomic Nucleus Atomic Number Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge may be approximated by the equation:. The shielding effect can be defined as a. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Screening effect/Shielding effect & Effective Nuclear charge kya h Define Shielding Effect And Effective Nuclear Charge The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The shielding effect explains why. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge is the net charge an electron. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube Define Shielding Effect And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The effective nuclear charge may be approximated by the equation:. The shielding effect can be defined as a reduction in the. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube Define Shielding Effect And Effective Nuclear Charge Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The shielding effect explains why. The effective nuclear charge may. Define Shielding Effect And Effective Nuclear Charge.
From mungfali.com
What Is Shielding Effect Define Shielding Effect And Effective Nuclear Charge The shielding effect explains why. The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The effective nuclear charge is the net charge an electron experiences in an atom with. Define Shielding Effect And Effective Nuclear Charge.
From www.studypool.com
SOLUTION Electron configurations of cations and anions shielding Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The concept of electron shielding, in which intervening electrons act to reduce the. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
IIT Shielding effect, Nuclear Charge, power, Atomic size Define Shielding Effect And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in. Define Shielding Effect And Effective Nuclear Charge.
From ar.inspiredpencil.com
Shielding Effect Electrons Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The. Define Shielding Effect And Effective Nuclear Charge.
From www.numerade.com
SOLVEDDefine shielding and effective nuclear charge. What is the Define Shielding Effect And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The effective nuclear charge may be approximated by the equation:. The shielding effect. Define Shielding Effect And Effective Nuclear Charge.
From fyozgirgf.blob.core.windows.net
Define Electron Shielding And Effective Nuclear Charge at Gloria Hou blog Define Shielding Effect And Effective Nuclear Charge Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The effective nuclear charge is. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Shielding Effect Easy way to Understand Define Shielding Effect And Effective Nuclear Charge The shielding effect explains why. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by. Define Shielding Effect And Effective Nuclear Charge.
From ar.inspiredpencil.com
Shielding Effect Electrons Define Shielding Effect And Effective Nuclear Charge The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a. Define Shielding Effect And Effective Nuclear Charge.
From byjus.com
What is effective nuclear charge and shielding effect? Define Shielding Effect And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear charge may be approximated by the equation:. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The shielding effect can be defined as a reduction in the effective nuclear. Define Shielding Effect And Effective Nuclear Charge.
From www.careerpower.in
Effective Nuclear Charge Define, Formula, Atomic Number Define Shielding Effect And Effective Nuclear Charge The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is. Define Shielding Effect And Effective Nuclear Charge.
From www.breakingatom.com
Nuclear Charge Define Shielding Effect And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud,. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Definition, Examples ,Trend ,Formula Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The shielding effect explains why. The effective nuclear charge may be approximated by the equation:. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Shielding effect and effective nuclear charge By Rohit sir YouTube Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The effective nuclear charge is the net charge an electron experiences in an. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Nuclear Charge and Effective Nuclear Charge YouTube Define Shielding Effect And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The. Define Shielding Effect And Effective Nuclear Charge.
From www.slideserve.com
PPT Quantum Theory PowerPoint Presentation, free download ID1185278 Define Shielding Effect And Effective Nuclear Charge The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The effective nuclear charge may be approximated by the equation:. The concept of. Define Shielding Effect And Effective Nuclear Charge.
From www.slideserve.com
PPT Atomic Radii PowerPoint Presentation, free download ID6448698 Define Shielding Effect And Effective Nuclear Charge The shielding effect explains why. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The effective nuclear charge may be approximated by the equation:. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction.. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge and the Shielding Effect YouTube Define Shielding Effect And Effective Nuclear Charge The shielding effect explains why. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The shielding effect describes the balance between the pull of. Define Shielding Effect And Effective Nuclear Charge.
From pediaa.com
What is the Difference Between Effective Nuclear Charge and Shielding Define Shielding Effect And Effective Nuclear Charge The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by. Define Shielding Effect And Effective Nuclear Charge.
From www.toppr.com
How are shielding effect and atomic radius related? Define Shielding Effect And Effective Nuclear Charge The shielding effect explains why. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction. The concept of electron shielding, in which intervening. Define Shielding Effect And Effective Nuclear Charge.
From www.youtube.com
Shielding effect, Effective Nuclear charge, effects YouTube Define Shielding Effect And Effective Nuclear Charge The concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The. Define Shielding Effect And Effective Nuclear Charge.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE Define Shielding Effect And Effective Nuclear Charge Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the reduction of the effective nuclear charge. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The shielding effect can be defined as a reduction in the effective nuclear. Define Shielding Effect And Effective Nuclear Charge.