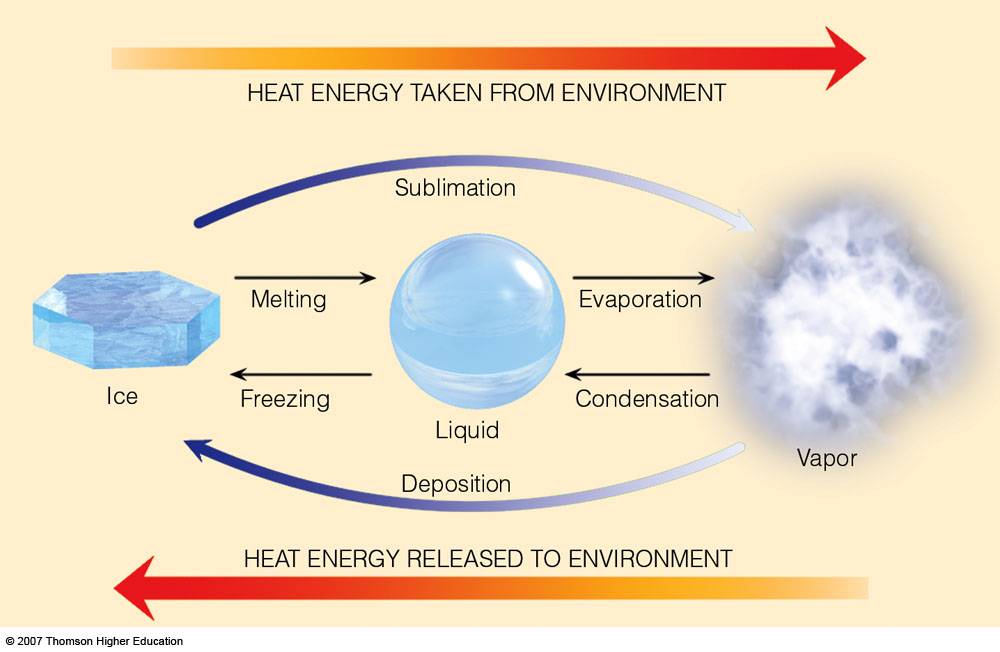
Does Evaporation Always Require Heat . In the absence of an external heat. Heat from the sun, or solar energy, powers the evaporation process. At higher elevations, air pressure is lower; Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. As water is heated, its vapour pressure. The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. When you’re boiling water on the stove, you’re adding heat to liquid water.
from apollo.nvu.vsc.edu
Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. As water is heated, its vapour pressure. In the absence of an external heat. Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. When you’re boiling water on the stove, you’re adding heat to liquid water. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. Heat from the sun, or solar energy, powers the evaporation process. At higher elevations, air pressure is lower;
Latent Heat of evaporation, fusion, and freezing
Does Evaporation Always Require Heat Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. In the absence of an external heat. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. Heat from the sun, or solar energy, powers the evaporation process. When you’re boiling water on the stove, you’re adding heat to liquid water. At higher elevations, air pressure is lower; The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. As water is heated, its vapour pressure. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at.
From www.aplustopper.com
How can we Separate a Mixture of a Solid and a Liquid using Evaporation Does Evaporation Always Require Heat Heat from the sun, or solar energy, powers the evaporation process. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. At higher elevations, air pressure is lower; As water is heated, its vapour pressure. Evaporation occurs when. Does Evaporation Always Require Heat.
From www.bbc.co.uk
Evaporation BBC Bitesize Does Evaporation Always Require Heat As water is heated, its vapour pressure. The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. Heat from the sun, or solar energy, powers the evaporation process. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. Evaporation can be assisted by adding heat, but. Does Evaporation Always Require Heat.
From www.slideserve.com
PPT CHAPTER 1 MATTER IN OUR SURROUNDINGS PowerPoint Presentation Does Evaporation Always Require Heat Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. In the absence of an external heat. At higher elevations, air. Does Evaporation Always Require Heat.
From www.geeksforgeeks.org
Separation by Evaporation Does Evaporation Always Require Heat When you’re boiling water on the stove, you’re adding heat to liquid water. The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. In the absence of an external heat. Heat from the sun, or solar energy, powers the evaporation process. As water is heated, its vapour pressure. Evaporation occurs when energy (heat). Does Evaporation Always Require Heat.
From stock.adobe.com
The mechanisms of heat transfer conduction, convection, radiation Does Evaporation Always Require Heat As water is heated, its vapour pressure. In the absence of an external heat. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. At higher elevations, air pressure is lower; Heat from the sun, or solar energy, powers the evaporation process. The energy gained during vaporization requires 2260 joules/gram, while the energy. Does Evaporation Always Require Heat.
From guides.co
Phase Change Requires Heat FD2021 Fundamentals of Fire and Does Evaporation Always Require Heat When you’re boiling water on the stove, you’re adding heat to liquid water. At higher elevations, air pressure is lower; Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. Heat from the sun, or solar energy, powers the evaporation process. The energy gained during vaporization requires 2260 joules/gram, while the energy gained. Does Evaporation Always Require Heat.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Does Evaporation Always Require Heat Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. As water is heated, its vapour pressure.. Does Evaporation Always Require Heat.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Does Evaporation Always Require Heat Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. The energy gained during vaporization requires 2260 joules/gram, while. Does Evaporation Always Require Heat.
From www.youtube.com
Evaporation causes cooling??? Explained YouTube Does Evaporation Always Require Heat The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. Heat from the sun, or solar energy, powers the evaporation process. Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break.. Does Evaporation Always Require Heat.
From www.pinterest.com
Conduction, convection and radiation are the three different modes of Does Evaporation Always Require Heat Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. Heat from the sun, or solar energy, powers the evaporation process. Since the air pressure can no longer overcome the vapour pressure. Does Evaporation Always Require Heat.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Does Evaporation Always Require Heat Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. At higher elevations, air pressure is lower; When you’re boiling water on the stove, you’re adding heat to liquid water. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. Evaporation can be assisted. Does Evaporation Always Require Heat.
From www.processtechacademy.com
Proc Tech & Oper Acad Sensible & Latent Heat Does Evaporation Always Require Heat The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. At higher elevations, air pressure is lower; As water is heated, its vapour pressure. Evaporation can be assisted by adding heat, but adding heat is not. Does Evaporation Always Require Heat.
From www.youtube.com
Biowork 6p137151 Heat Transfer, Evaporation, Condensation, Stripping Does Evaporation Always Require Heat The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. When you’re boiling water on the stove, you’re adding. Does Evaporation Always Require Heat.
From www.howmechanismworks.com
HowMechanismWorks ? Modes Of Heat Transfer Does Evaporation Always Require Heat It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. When you’re boiling water on the stove, you’re adding heat to liquid water. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually. Does Evaporation Always Require Heat.
From thepicturespatch.blogspot.com
Evaporate The Physics Of Evaporation Explained Pressure Is The Key Does Evaporation Always Require Heat Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. When you’re boiling water on the stove, you’re adding heat to liquid water. At higher elevations, air pressure is lower; The energy gained during vaporization requires. Does Evaporation Always Require Heat.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Evaporation Always Require Heat At higher elevations, air pressure is lower; Heat from the sun, or solar energy, powers the evaporation process. When you’re boiling water on the stove, you’re adding heat to liquid water. As water is heated, its vapour pressure. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. Evaporation, process by which an. Does Evaporation Always Require Heat.
From www.youtube.com
Why evaporation is a cooling process? YouTube Does Evaporation Always Require Heat It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. At higher elevations, air pressure is lower; Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break.. Does Evaporation Always Require Heat.
From www.247localhvac.com
What is a Heat Pump & How Does It Work? Does Evaporation Always Require Heat It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. In the absence of an external heat. At higher elevations, air pressure is lower; Heat from the sun, or solar energy, powers the evaporation. Does Evaporation Always Require Heat.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Evaporation Always Require Heat Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. When you’re boiling water on the stove, you’re adding heat to liquid water. As water is heated, its vapour pressure. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. The energy gained during vaporization requires 2260 joules/gram,. Does Evaporation Always Require Heat.
From www.teachoo.com
Latent Heat of Vaporization and Fusion Definition Teachoo Does Evaporation Always Require Heat Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. At higher elevations, air pressure is lower; The energy gained during. Does Evaporation Always Require Heat.
From brainly.in
how does evaporation cause cooling?? Brainly.in Does Evaporation Always Require Heat Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Heat from the sun, or solar energy, powers the evaporation process. When you’re boiling water on the stove, you’re adding heat to liquid water.. Does Evaporation Always Require Heat.
From www.youtube.com
Thermal Evaporation What is it and how does it work? YouTube Does Evaporation Always Require Heat Heat from the sun, or solar energy, powers the evaporation process. Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. When you’re boiling water on the stove, you’re adding heat to liquid water. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at.. Does Evaporation Always Require Heat.
From www.slideserve.com
PPT Ch. 27 Evaporation, Condensation, and Precipitation PowerPoint Does Evaporation Always Require Heat At higher elevations, air pressure is lower; Heat from the sun, or solar energy, powers the evaporation process. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. When you’re boiling water on the stove, you’re adding heat to liquid water. The energy gained during vaporization requires 2260 joules/gram, while the. Does Evaporation Always Require Heat.
From www.youtube.com
Evaporation Heat transfer coeff (DRAFT video) YouTube Does Evaporation Always Require Heat Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. The substantial energy requirement for evaporation is due to one of. Does Evaporation Always Require Heat.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Does Evaporation Always Require Heat Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. At higher elevations, air pressure is lower; Heat from the sun, or solar energy, powers the evaporation process. The substantial energy requirement for evaporation is due to one. Does Evaporation Always Require Heat.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID2061272 Does Evaporation Always Require Heat The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. In the absence of an external heat. As water is heated, its vapour pressure. Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. It soaks up moisture from soil in a garden, as well as. Does Evaporation Always Require Heat.
From apollo.nvu.vsc.edu
Latent Heat of evaporation, fusion, and freezing Does Evaporation Always Require Heat The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. Evaporation, process by which an element or compound transitions from its. Does Evaporation Always Require Heat.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo Does Evaporation Always Require Heat Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. As water is heated, its vapour pressure. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. Heat from. Does Evaporation Always Require Heat.
From www.slideserve.com
PPT The four horsemen of heat transfer C ontents Conduction Does Evaporation Always Require Heat At higher elevations, air pressure is lower; Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. It soaks up moisture from soil in a garden, as well as the biggest oceans and lakes. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at.. Does Evaporation Always Require Heat.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID Does Evaporation Always Require Heat The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. Heat from the sun, or solar energy, powers the evaporation process. Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. The energy gained during vaporization requires 2260 joules/gram, while the energy gained during. Does Evaporation Always Require Heat.
From abhimanyusir.blogspot.com
Geography GK, Notes, Maps, Current Affairs, and NEWS for All Classes Does Evaporation Always Require Heat In the absence of an external heat. Heat from the sun, or solar energy, powers the evaporation process. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. When you’re boiling water on the stove, you’re adding heat to liquid water. As water is heated, its vapour pressure. Evaporation, process by which an element or. Does Evaporation Always Require Heat.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Does Evaporation Always Require Heat When you’re boiling water on the stove, you’re adding heat to liquid water. Since the air pressure can no longer overcome the vapour pressure of the water, the water boils. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. It soaks up moisture from soil in a garden, as well. Does Evaporation Always Require Heat.
From www.teachoo.com
How does Evaporation cause cooling? Explain (with Examples) Teachoo Does Evaporation Always Require Heat Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. Evaporation can be assisted by. Does Evaporation Always Require Heat.
From sciencenotes.org
Evaporative Cooler How It Works and Examples Does Evaporation Always Require Heat The substantial energy requirement for evaporation is due to one of water’s peculiar properties—its unusually high latent heat of. Evaporation can be assisted by adding heat, but adding heat is not required for evaporation. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. As water is heated, its vapour pressure. The energy gained during. Does Evaporation Always Require Heat.
From collegedunia.com
Factor Affecting Evaporation Definition, Rate of Evaporation, Examples Does Evaporation Always Require Heat When you’re boiling water on the stove, you’re adding heat to liquid water. The energy gained during vaporization requires 2260 joules/gram, while the energy gained during melting is only 334. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. At higher elevations, air pressure is lower; Evaporation can. Does Evaporation Always Require Heat.