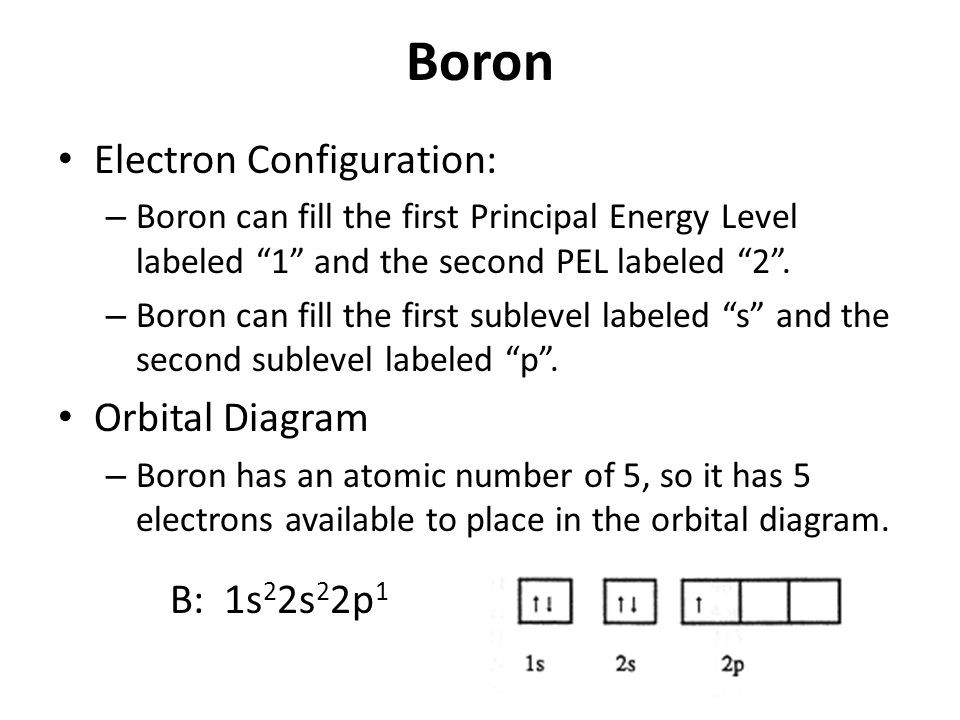
Why Is Boron Stable With 6 Electrons . The reason for this exception lies in the atomic. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). You may assume the valences of the chemical elements—the number of electrons with which. 93 rows updated on may 19, 2024. The boron shares its three electrons with three fluorine atoms. The fluorine atoms follow the octet rule, but boron has only six. However, boron only needs six valence electrons to achieve a stable electron configuration. Boron's configuration, however, is $1s^2 2s^2 2p^1$. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position.
from periodictable.me
93 rows updated on may 19, 2024. The fluorine atoms follow the octet rule, but boron has only six. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. However, boron only needs six valence electrons to achieve a stable electron configuration. Boron's configuration, however, is $1s^2 2s^2 2p^1$. The boron shares its three electrons with three fluorine atoms. You may assume the valences of the chemical elements—the number of electrons with which. The reason for this exception lies in the atomic. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position.
How To Find The Electron Configuration For Boron Dynamic Periodic
Why Is Boron Stable With 6 Electrons 93 rows updated on may 19, 2024. Boron's configuration, however, is $1s^2 2s^2 2p^1$. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The fluorine atoms follow the octet rule, but boron has only six. The boron shares its three electrons with three fluorine atoms. However, boron only needs six valence electrons to achieve a stable electron configuration. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. The reason for this exception lies in the atomic. You may assume the valences of the chemical elements—the number of electrons with which. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). 93 rows updated on may 19, 2024.
From ar.inspiredpencil.com
Boron Protons Neutrons Electrons Why Is Boron Stable With 6 Electrons The boron shares its three electrons with three fluorine atoms. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. The reason for this exception lies in the atomic. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. 93 rows updated on may 19, 2024.. Why Is Boron Stable With 6 Electrons.
From www.youtube.com
Why Boron is Electron Deficient in Boron Triflouride (BF3) ? YouTube Why Is Boron Stable With 6 Electrons Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). Boron's configuration, however, is $1s^2 2s^2 2p^1$. The fluorine atoms follow the octet rule, but boron. Why Is Boron Stable With 6 Electrons.
From www.chemistrylearner.com
Exception to the Octet Rule Examples Why Is Boron Stable With 6 Electrons You may assume the valences of the chemical elements—the number of electrons with which. The reason for this exception lies in the atomic. Boron's configuration, however, is $1s^2 2s^2 2p^1$. The fluorine atoms follow the octet rule, but boron has only six. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in. Why Is Boron Stable With 6 Electrons.
From www.doubtnut.com
Why boron forms electron deficient compounds? Why Is Boron Stable With 6 Electrons Boron's configuration, however, is $1s^2 2s^2 2p^1$. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. You may assume the valences of the chemical elements—the number of electrons with which. The fluorine atoms follow the octet rule, but boron has only six. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6. Why Is Boron Stable With 6 Electrons.
From ar.inspiredpencil.com
Boron Symbol On Periodic Table Why Is Boron Stable With 6 Electrons Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. The reason for this exception lies in the atomic. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with. Why Is Boron Stable With 6 Electrons.
From mungfali.com
Lewis Electron Dot Structure For Boron Why Is Boron Stable With 6 Electrons The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). The boron shares its three electrons with three fluorine atoms. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. 93 rows updated on may 19,. Why Is Boron Stable With 6 Electrons.
From stock.adobe.com
Boron element with symbol B and atomic number 5.Isolated molecular Why Is Boron Stable With 6 Electrons The fluorine atoms follow the octet rule, but boron has only six. 93 rows updated on may 19, 2024. The boron shares its three electrons with three fluorine atoms. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron).. Why Is Boron Stable With 6 Electrons.
From www.pinterest.com.au
boron atom Google Search Lasbril, Auteurs, Afbeeldingen Why Is Boron Stable With 6 Electrons You may assume the valences of the chemical elements—the number of electrons with which. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. The reason for this exception lies in the atomic. The fluorine atoms follow the octet rule, but boron has only six. Boron's configuration, however, is. Why Is Boron Stable With 6 Electrons.
From ar.inspiredpencil.com
Boron Protons Neutrons Electrons Why Is Boron Stable With 6 Electrons You may assume the valences of the chemical elements—the number of electrons with which. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). However, boron only needs six valence electrons to achieve a stable electron configuration. Decoding boron's. Why Is Boron Stable With 6 Electrons.
From www.animalia-life.club
Boron Protons Neutrons Electrons Why Is Boron Stable With 6 Electrons The boron shares its three electrons with three fluorine atoms. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The reason for this exception lies in the atomic. Boron's configuration, however, is $1s^2 2s^2 2p^1$. The fluorine atoms follow the octet rule, but boron has only six. 93 rows updated on may 19, 2024. You may. Why Is Boron Stable With 6 Electrons.
From 9to5science.com
[Solved] Why does Boron only need 6 valence electrons 9to5Science Why Is Boron Stable With 6 Electrons Boron's configuration, however, is $1s^2 2s^2 2p^1$. However, boron only needs six valence electrons to achieve a stable electron configuration. The boron shares its three electrons with three fluorine atoms. You may assume the valences of the chemical elements—the number of electrons with which. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The fluorine that. Why Is Boron Stable With 6 Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Boron Have? Why Is Boron Stable With 6 Electrons The fluorine atoms follow the octet rule, but boron has only six. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. You may assume the valences of the chemical elements—the number of electrons with which. However, boron only needs six valence electrons to achieve a stable electron configuration.. Why Is Boron Stable With 6 Electrons.
From wireenginedominators.z14.web.core.windows.net
Boron Electron Dot Diagram Why Is Boron Stable With 6 Electrons You may assume the valences of the chemical elements—the number of electrons with which. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. However, boron only needs six valence electrons to achieve a stable electron configuration. Boron's configuration, however, is $1s^2 2s^2 2p^1$. The boron shares its three electrons with three fluorine atoms. 93 rows updated. Why Is Boron Stable With 6 Electrons.
From borates.today
Boron Electron Valence Borates Today Why Is Boron Stable With 6 Electrons Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. You may assume the valences of the chemical elements—the number of electrons with which. The boron shares its three electrons with three fluorine atoms. The reason for this exception lies in the atomic. The fluorine that shares a double. Why Is Boron Stable With 6 Electrons.
From periodictableguide.com
Boron (B) Periodic Table (Element Information & More) Why Is Boron Stable With 6 Electrons The fluorine atoms follow the octet rule, but boron has only six. The reason for this exception lies in the atomic. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. You may assume the valences of the chemical elements—the number of electrons with which. Boron's configuration, however, is $1s^2 2s^2 2p^1$. However, boron only needs six. Why Is Boron Stable With 6 Electrons.
From www.animalia-life.club
Boron Protons Neutrons Electrons Why Is Boron Stable With 6 Electrons The fluorine atoms follow the octet rule, but boron has only six. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. 93 rows updated on may 19, 2024. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs. Why Is Boron Stable With 6 Electrons.
From courses.lumenlearning.com
Electrons Biology for Majors I Why Is Boron Stable With 6 Electrons 93 rows updated on may 19, 2024. Boron's configuration, however, is $1s^2 2s^2 2p^1$. However, boron only needs six valence electrons to achieve a stable electron configuration. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. The reason for this exception lies in the atomic. You may assume. Why Is Boron Stable With 6 Electrons.
From h-o-m-e.org
Boron Trihydride (BH3) The Exception to the Octet Rule Why Is Boron Stable With 6 Electrons However, boron only needs six valence electrons to achieve a stable electron configuration. Boron's configuration, however, is $1s^2 2s^2 2p^1$. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. The fluorine atoms follow the octet rule, but boron has only six. You may assume the valences of the. Why Is Boron Stable With 6 Electrons.
From periodictable.me
How To Find The Electron Configuration For Boron Dynamic Periodic Why Is Boron Stable With 6 Electrons The boron shares its three electrons with three fluorine atoms. The fluorine atoms follow the octet rule, but boron has only six. Boron's configuration, however, is $1s^2 2s^2 2p^1$. However, boron only needs six valence electrons to achieve a stable electron configuration. You may assume the valences of the chemical elements—the number of electrons with which. The fluorine that shares. Why Is Boron Stable With 6 Electrons.
From www.researchgate.net
Boroncentered stable radical anion/radical cation pair. Reproduced Why Is Boron Stable With 6 Electrons Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The reason for this exception lies in the atomic. Boron's configuration, however, is $1s^2 2s^2 2p^1$. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. You may assume the valences of the chemical elements—the number. Why Is Boron Stable With 6 Electrons.
From ar.inspiredpencil.com
Electron Configuration For Boron Why Is Boron Stable With 6 Electrons However, boron only needs six valence electrons to achieve a stable electron configuration. The reason for this exception lies in the atomic. The fluorine atoms follow the octet rule, but boron has only six. The boron shares its three electrons with three fluorine atoms. The fluorine that shares a double bond with boron has six electrons around it (four from. Why Is Boron Stable With 6 Electrons.
From www.reddit.com
why is boron’s net charge 0? r/chemhelp Why Is Boron Stable With 6 Electrons However, boron only needs six valence electrons to achieve a stable electron configuration. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The boron shares its three electrons with three fluorine atoms. The reason for this exception lies in the atomic. Boron's configuration, however, is $1s^2 2s^2 2p^1$. Decoding boron's electron preferences • boron's electrons •. Why Is Boron Stable With 6 Electrons.
From byjus.com
Why boron is considered as non metal since it has three electrons in Why Is Boron Stable With 6 Electrons The fluorine atoms follow the octet rule, but boron has only six. 93 rows updated on may 19, 2024. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). You may assume the valences of the chemical elements—the number. Why Is Boron Stable With 6 Electrons.
From www.nuclear-power.com
Boron Electron Affinity Electronegativity Ionization Energy of Why Is Boron Stable With 6 Electrons The fluorine atoms follow the octet rule, but boron has only six. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). Boron's configuration, however, is $1s^2 2s^2 2p^1$. However, boron only needs six valence electrons to achieve a. Why Is Boron Stable With 6 Electrons.
From www.animalia-life.club
Boron Protons Neutrons Electrons Why Is Boron Stable With 6 Electrons However, boron only needs six valence electrons to achieve a stable electron configuration. The reason for this exception lies in the atomic. You may assume the valences of the chemical elements—the number of electrons with which. The fluorine atoms follow the octet rule, but boron has only six. The boron shares its three electrons with three fluorine atoms. The fluorine. Why Is Boron Stable With 6 Electrons.
From www.youtube.com
How to find Protons and Electrons for B 3+ (Boron Ion) YouTube Why Is Boron Stable With 6 Electrons You may assume the valences of the chemical elements—the number of electrons with which. Boron's configuration, however, is $1s^2 2s^2 2p^1$. 93 rows updated on may 19, 2024. The fluorine atoms follow the octet rule, but boron has only six. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique. Why Is Boron Stable With 6 Electrons.
From borates.today
Alkaline Boron A Balancing Force In Nature Why Is Boron Stable With 6 Electrons Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The fluorine atoms follow the octet rule, but boron has only six. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). Decoding boron's electron preferences. Why Is Boron Stable With 6 Electrons.
From www.alamy.com
Symbol and electron diagram Boron illustration Stock Vector Image & Art Why Is Boron Stable With 6 Electrons The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). You may assume the valences of the chemical elements—the number of electrons with which. Boron's configuration, however, is $1s^2 2s^2 2p^1$. Having 6 valence electrons would mean having a. Why Is Boron Stable With 6 Electrons.
From signalduo.com
Top 7 how many electrons and neutrons does boron have 2022 Why Is Boron Stable With 6 Electrons Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. However, boron only needs six valence electrons to achieve a stable electron configuration. The boron shares its three electrons with three fluorine atoms. The reason for this exception lies in the atomic. Boron's configuration, however, is $1s^2 2s^2 2p^1$.. Why Is Boron Stable With 6 Electrons.
From jetestelavirb.com
Electron Dot Structure For Boron Art & Bussines Why Is Boron Stable With 6 Electrons The reason for this exception lies in the atomic. You may assume the valences of the chemical elements—the number of electrons with which. The fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of electrons and one each from its two bonds with boron). Boron's configuration, however, is $1s^2 2s^2. Why Is Boron Stable With 6 Electrons.
From www.dreamstime.com
Boron stock illustration. Illustration of symbol, electrons 89682672 Why Is Boron Stable With 6 Electrons The reason for this exception lies in the atomic. You may assume the valences of the chemical elements—the number of electrons with which. The fluorine atoms follow the octet rule, but boron has only six. However, boron only needs six valence electrons to achieve a stable electron configuration. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2. Why Is Boron Stable With 6 Electrons.
From galvinconanstuart.blogspot.com
Electron Dot Diagram For Boron General Wiring Diagram Why Is Boron Stable With 6 Electrons Boron's configuration, however, is $1s^2 2s^2 2p^1$. The boron shares its three electrons with three fluorine atoms. The reason for this exception lies in the atomic. 93 rows updated on may 19, 2024. The fluorine atoms follow the octet rule, but boron has only six. The fluorine that shares a double bond with boron has six electrons around it (four. Why Is Boron Stable With 6 Electrons.
From schematron.org
Lewis Dot Diagram For Boron Wiring Diagram Pictures Why Is Boron Stable With 6 Electrons The reason for this exception lies in the atomic. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The fluorine atoms follow the octet rule, but boron has only six. Boron's configuration, however, is $1s^2. Why Is Boron Stable With 6 Electrons.
From techiescientist.com
Boron Bohr Model Diagram, Steps To Draw Techiescientist Why Is Boron Stable With 6 Electrons However, boron only needs six valence electrons to achieve a stable electron configuration. Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The boron shares its three electrons with three fluorine atoms. 93 rows updated on may 19, 2024. You may assume the valences of the chemical elements—the number of electrons with which. The fluorine that. Why Is Boron Stable With 6 Electrons.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table Why Is Boron Stable With 6 Electrons Having 6 valence electrons would mean having a configuration of $1s^2 2s^2 2p^4$. The boron shares its three electrons with three fluorine atoms. Decoding boron's electron preferences • boron's electrons • discover why boron strives for 6 electrons, finding stability in its unique position. 93 rows updated on may 19, 2024. However, boron only needs six valence electrons to achieve. Why Is Boron Stable With 6 Electrons.