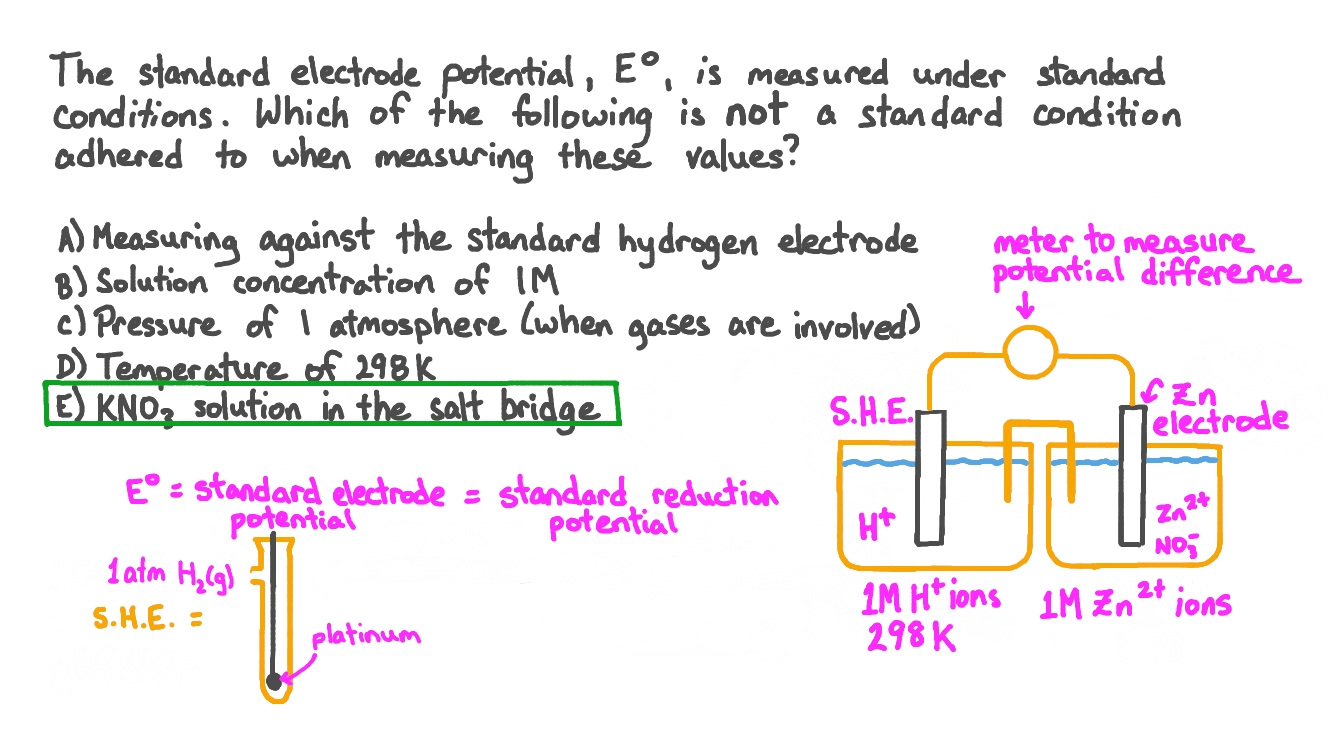
What Does Negative Electrode Potential Mean . The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. The pt electrode in the permanganate solution is the cathode; The equilibrium position lies more to the left. Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The one in the tin solution is the anode. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur.
from mungfali.com
The pt electrode in the permanganate solution is the cathode; The equilibrium position lies more to the left. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The one in the tin solution is the anode. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),.
Electrode Potential Series
What Does Negative Electrode Potential Mean In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The equilibrium position lies more to the left. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. The one in the tin solution is the anode. Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The pt electrode in the permanganate solution is the cathode; In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be.
From howtomechatronics.com
Electric Potential and Electric Potential Difference (Voltage) How To What Does Negative Electrode Potential Mean The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The pt electrode in the permanganate solution is the cathode; The equilibrium. What Does Negative Electrode Potential Mean.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students What Does Negative Electrode Potential Mean The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The pt electrode in the permanganate solution is the cathode; The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. Negative values. What Does Negative Electrode Potential Mean.
From www.youtube.com
Difference between Oxidation potential and Reduction potential What Does Negative Electrode Potential Mean In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text. What Does Negative Electrode Potential Mean.
From www.researchgate.net
How the shape of the voltage profile of the negative electrode (NE) can What Does Negative Electrode Potential Mean The equilibrium position lies more to the left. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. The more negative. What Does Negative Electrode Potential Mean.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 What Does Negative Electrode Potential Mean Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. The more negative (or less positive) the electrode potential, the less likely. What Does Negative Electrode Potential Mean.
From www.researchgate.net
Positive and negative electrode potential profiles from a NCM523/Gr What Does Negative Electrode Potential Mean The equilibrium position lies more to the left. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The pt electrode in the permanganate solution is the. What Does Negative Electrode Potential Mean.
From www.slideserve.com
PPT Electric Potential PowerPoint Presentation, free download ID What Does Negative Electrode Potential Mean In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The equilibrium position lies more to the left. Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The pt electrode in the permanganate solution is the. What Does Negative Electrode Potential Mean.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 What Does Negative Electrode Potential Mean The one in the tin solution is the anode. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the. What Does Negative Electrode Potential Mean.
From slideplayer.com
Redox Recap. ppt download What Does Negative Electrode Potential Mean The one in the tin solution is the anode. Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The pt electrode in the permanganate solution is the. What Does Negative Electrode Potential Mean.
From quizlet.com
ELECTRODE POTENTIAL AND CELL Diagram Quizlet What Does Negative Electrode Potential Mean The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The one in the tin solution is the anode. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. The equilibrium position. What Does Negative Electrode Potential Mean.
From www.slideserve.com
PPT Electric Potential PowerPoint Presentation, free download ID What Does Negative Electrode Potential Mean The pt electrode in the permanganate solution is the cathode; In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. The. What Does Negative Electrode Potential Mean.
From www.researchgate.net
Negative electrode potential vs. chargedischarge capacity, first C/10 What Does Negative Electrode Potential Mean The equilibrium position lies more to the left. The one in the tin solution is the anode. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and. What Does Negative Electrode Potential Mean.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision What Does Negative Electrode Potential Mean The equilibrium position lies more to the left. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The one in the tin. What Does Negative Electrode Potential Mean.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive What Does Negative Electrode Potential Mean The one in the tin solution is the anode. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. The pt electrode in the permanganate solution is the cathode; The more negative (or less positive) the electrode potential, the less likely it is that reduction. What Does Negative Electrode Potential Mean.
From www.youtube.com
What does electrode potential mean? YouTube What Does Negative Electrode Potential Mean The pt electrode in the permanganate solution is the cathode; The one in the tin solution is the anode. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. The equilibrium position lies more to the left. Although it is impossible to measure the potential. What Does Negative Electrode Potential Mean.
From mungfali.com
Electrode Potential Series What Does Negative Electrode Potential Mean The pt electrode in the permanganate solution is the cathode; In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The potential of an electrochemical cell is. What Does Negative Electrode Potential Mean.
From app.pandai.org
Standard Electrode Potential What Does Negative Electrode Potential Mean In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The equilibrium position lies more to the left. The more negative (or less positive) the electrode potential,. What Does Negative Electrode Potential Mean.
From www.researchgate.net
Voltage and current profile of the cell and the negative electrode What Does Negative Electrode Potential Mean Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. The equilibrium position lies more to the left. Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The potential of an electrochemical cell. What Does Negative Electrode Potential Mean.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts What Does Negative Electrode Potential Mean The equilibrium position lies more to the left. The one in the tin solution is the anode. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v. What Does Negative Electrode Potential Mean.
From www.britannica.com
Electric potential Definition, Facts, & Units Britannica What Does Negative Electrode Potential Mean The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. The pt electrode in the permanganate solution is the cathode; Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of. What Does Negative Electrode Potential Mean.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Does Negative Electrode Potential Mean Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The more negative (or less positive) the electrode potential, the less likely it is that reduction of. What Does Negative Electrode Potential Mean.
From greenenergymaterial.com
What Is Electrode Potential And Their Applications » Green Energy Material What Does Negative Electrode Potential Mean The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The one in the tin solution is the anode. The pt electrode in the permanganate solution is. What Does Negative Electrode Potential Mean.
From facts.net
16 Unbelievable Facts About Standard Electrode Potential What Does Negative Electrode Potential Mean Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The potential of an electrochemical cell is the difference between the potential at the. What Does Negative Electrode Potential Mean.
From www.comsol.com
Does the Current Flow Backwards Inside a Battery? COMSOL Blog What Does Negative Electrode Potential Mean The one in the tin solution is the anode. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The equilibrium position lies more to the left. Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The. What Does Negative Electrode Potential Mean.
From www.researchgate.net
(a) Cell voltage, (b) positive electrode potential and (c) negative What Does Negative Electrode Potential Mean Although it is impossible to measure the potential of any electrode directly, we can choose a reference electrode whose potential is. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. The one in the tin solution is the anode. The equilibrium position. What Does Negative Electrode Potential Mean.
From www.slideshare.net
Lecture21 potential What Does Negative Electrode Potential Mean The pt electrode in the permanganate solution is the cathode; The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text {anode}\),. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The. What Does Negative Electrode Potential Mean.
From www.researchgate.net
Change of the potential of the negative electrode (multiplied by minus What Does Negative Electrode Potential Mean The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The one in the tin solution is the anode. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. Although it is impossible to measure the potential. What Does Negative Electrode Potential Mean.
From exowfmfux.blob.core.windows.net
Standard Electrode Examples at Lucille Fitzsimmons blog What Does Negative Electrode Potential Mean The one in the tin solution is the anode. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The equilibrium position lies more to the left.. What Does Negative Electrode Potential Mean.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Does Negative Electrode Potential Mean In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The one in the tin solution is the anode. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. Although it is impossible to measure the potential. What Does Negative Electrode Potential Mean.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog What Does Negative Electrode Potential Mean Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. The one in the tin solution is the anode. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. In electrochemistry, electrode potential is. What Does Negative Electrode Potential Mean.
From sop4cv.com
Electrochemical Potential What Does Negative Electrode Potential Mean The equilibrium position lies more to the left. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The pt electrode in the permanganate solution is the cathode; The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the. What Does Negative Electrode Potential Mean.
From www.youtube.com
Using Standard Electrode Potentials YouTube What Does Negative Electrode Potential Mean Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. The pt electrode in the permanganate solution is the cathode; The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The equilibrium position lies. What Does Negative Electrode Potential Mean.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts What Does Negative Electrode Potential Mean In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be. The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. The equilibrium position lies more to the left. The one in the tin solution is the anode.. What Does Negative Electrode Potential Mean.
From www.slideserve.com
PPT Electric Potential and Capacitance PowerPoint Presentation, free What Does Negative Electrode Potential Mean The one in the tin solution is the anode. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. The potential of an electrochemical cell is the difference between the potential at the cathode, \ (e_\text {cathode}\), and the potential at the anode, \ (e_\text. What Does Negative Electrode Potential Mean.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? What Does Negative Electrode Potential Mean The more negative (or less positive) the electrode potential, the less likely it is that reduction of that species will occur. Negative values for electrode potentials are simply a consequence of assigning a value of 0 v to the she, indicating the reactant of the half. In electrochemistry, electrode potential is the voltage of a galvanic cell built from a. What Does Negative Electrode Potential Mean.