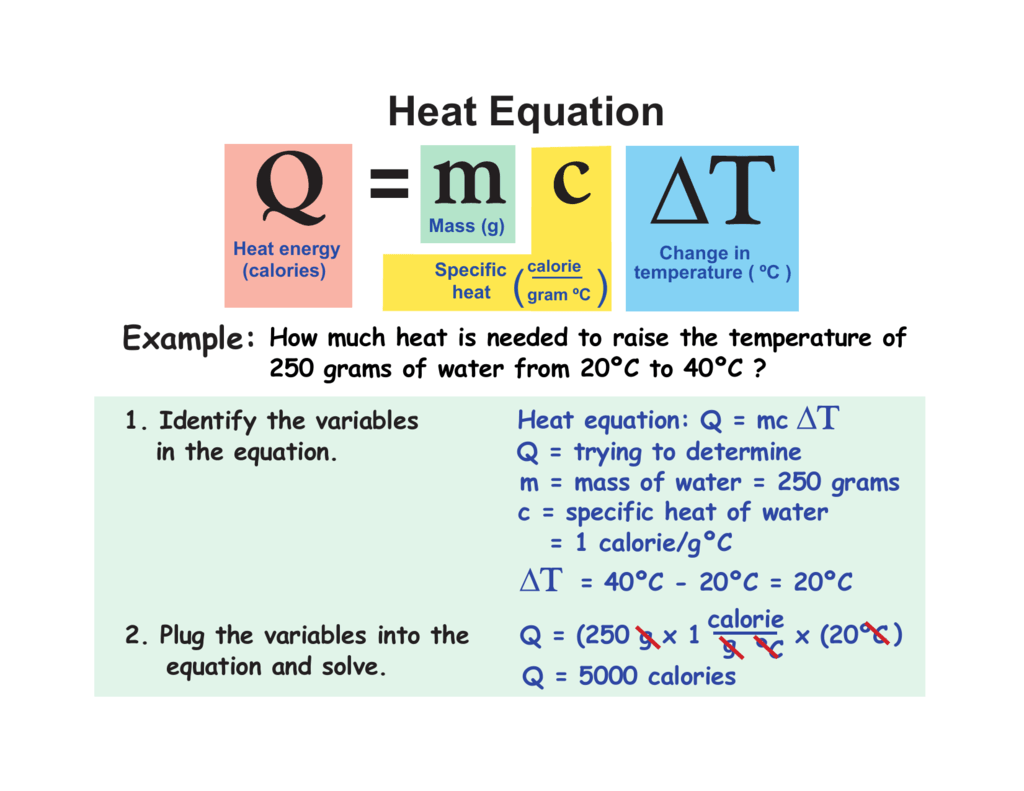
Calorimetry Final Temperature Formula . in calorimetry, the final temperature can be calculated using the formula: — compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. consequently, you can use this information to write the following equation: what is the final temperature of water and iron if a $\pu{30 g}$ piece of iron at $\pu{144 °c}$ was dropped into a calorimeter with. when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate. Reaction enthalpy = (heat capacity of contents) x. M is the mass of the substance. First, write down everything you know about each object and what you. — to solve calorimetry problems, you need to follow a simple instruction: find the initial and final temperature as well as the mass of the sample and energy supplied. this chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. — in this video, i show you how to solve for final temperature using. The letter q is the heat transferred in an exchange in calories, m. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. — this example problem demonstrates how to calculate the final temperature of a substance when given.
from www.slidemake.com
calorimetry is used to measure amounts of heat transferred to or from a substance. — this example problem demonstrates how to calculate the final temperature of a substance when given. To do so, the heat is exchanged with a. find the initial and final temperature as well as the mass of the sample and energy supplied. M is the mass of the substance. — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. — in this video, i show you how to solve for final temperature using. The letter q is the heat transferred in an exchange in calories, m. Assuming that all heat transfer occurs. — t_f=q/(mc)+t_i q=mcdeltat deltat is change in temperature, so we can rewrite the equation as:
Einstein Theory Of Specific Heat Presentation
Calorimetry Final Temperature Formula what is the final temperature of water and iron if a $\pu{30 g}$ piece of iron at $\pu{144 °c}$ was dropped into a calorimeter with. Assuming that all heat transfer occurs. Q signifies the heat energy transferred. when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate. this chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. The letter q is the heat transferred in an exchange in calories, m. — this example problem demonstrates how to calculate the final temperature of a substance when given. To do so, the heat is exchanged with a. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. calorimetry is used to measure amounts of heat transferred to or from a substance. what is the final temperature of water and iron if a $\pu{30 g}$ piece of iron at $\pu{144 °c}$ was dropped into a calorimeter with. find the initial and final temperature as well as the mass of the sample and energy supplied. Reaction enthalpy = (heat capacity of contents) x. in calorimetry, the final temperature can be calculated using the formula: — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. — in this video, i show you how to solve for final temperature using.
From www.slidemake.com
Einstein Theory Of Specific Heat Presentation Calorimetry Final Temperature Formula The letter q is the heat transferred in an exchange in calories, m. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. — in this video, i show you how to solve for final temperature using. use the equation for heat transfer \(q. Calorimetry Final Temperature Formula.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Final Temperature Formula — to solve calorimetry problems, you need to follow a simple instruction: M is the mass of the substance. — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. First, write down everything you know about each object and what you. To do so, the heat is exchanged with a.. Calorimetry Final Temperature Formula.
From www.youtube.com
change in temperature calculations YouTube Calorimetry Final Temperature Formula this chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. consequently, you can use this information to write the following equation: use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific. Calorimetry Final Temperature Formula.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Calorimetry Final Temperature Formula — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. — t_f=q/(mc)+t_i q=mcdeltat deltat is change in temperature, so we can rewrite the equation as: what is the final temperature of water and iron if a $\pu{30 g}$ piece of iron at $\pu{144 °c}$ was dropped into a calorimeter. Calorimetry Final Temperature Formula.
From quizdbpharmacies.z4.web.core.windows.net
How To Calculate Equilibrium Temperature Calorimetry Final Temperature Formula First, write down everything you know about each object and what you. when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate. Assuming that all heat transfer occurs. Subtract the final and initial. — t_f=q/(mc)+t_i q=mcdeltat deltat is change in temperature, so we can rewrite the equation as: the. Calorimetry Final Temperature Formula.
From quizspattering.z21.web.core.windows.net
How To Calculate Final Temperature Calorimetry Final Temperature Formula consequently, you can use this information to write the following equation: the calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a. in calorimetry, the final temperature can be calculated using the formula: this chemistry video tutorial explains how to find the final temperature in common. Calorimetry Final Temperature Formula.
From 2012books.lardbucket.org
Calorimetry Calorimetry Final Temperature Formula this chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. — this example problem demonstrates how to calculate the final temperature of a substance when given. Q signifies the heat energy transferred.. Calorimetry Final Temperature Formula.
From quizunderfires.z21.web.core.windows.net
Units Of Calorimeter Constant Calorimetry Final Temperature Formula — t_f=q/(mc)+t_i q=mcdeltat deltat is change in temperature, so we can rewrite the equation as: — compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The letter q is the heat transferred in an exchange in calories, m. — this example problem demonstrates how to calculate the final temperature of. Calorimetry Final Temperature Formula.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimetry Final Temperature Formula M is the mass of the substance. consequently, you can use this information to write the following equation: find the initial and final temperature as well as the mass of the sample and energy supplied. when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate. — t_f=q/(mc)+t_i q=mcdeltat. Calorimetry Final Temperature Formula.
From educationiciest.z21.web.core.windows.net
How To Calculate Thermal Equilibrium Calorimetry Final Temperature Formula this chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat. Calorimetry Final Temperature Formula.
From quizunderfires.z21.web.core.windows.net
Calorimeter Constant Calculator Online Calorimetry Final Temperature Formula Reaction enthalpy = (heat capacity of contents) x. Subtract the final and initial. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. calorimetry is used to measure amounts of heat transferred to or from. Calorimetry Final Temperature Formula.
From quizspattering.z21.web.core.windows.net
How To Calculate Final Temperature Calorimetry Final Temperature Formula Subtract the final and initial. The letter q is the heat transferred in an exchange in calories, m. consequently, you can use this information to write the following equation: this chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Q signifies the heat energy transferred. Reaction enthalpy = (heat capacity of. Calorimetry Final Temperature Formula.
From www.numerade.com
SOLVED Find the heat lost by the water, calorimeter cup (c = 0.21 Calorimetry Final Temperature Formula — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. Reaction enthalpy = (heat capacity of contents) x. what is the final temperature of water and iron if a $\pu{30 g}$ piece of iron at $\pu{144 °c}$ was dropped into a calorimeter with. consequently, you can use this information. Calorimetry Final Temperature Formula.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Final Temperature Formula this chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. Assuming that all heat transfer occurs. — this example problem demonstrates how to calculate the final temperature of a substance when given. — to solve calorimetry problems, you need to follow a simple instruction: in calorimetry, the final temperature. Calorimetry Final Temperature Formula.
From www.youtube.com
Solving for Final Temperature in Calorimetry YouTube Calorimetry Final Temperature Formula Reaction enthalpy = (heat capacity of contents) x. Subtract the final and initial. — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. — compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Assuming that all heat transfer occurs. — to solve. Calorimetry Final Temperature Formula.
From worksheetaidansuskm.z13.web.core.windows.net
Write The Formula To Explain Specific Heat Calorimetry Final Temperature Formula — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. use the equation for heat transfer q = m c δ t q = m c δ t to express the heat transferred from the pan in terms of the mass of the. Assuming that all heat transfer occurs. Reaction. Calorimetry Final Temperature Formula.
From www.bartleby.com
Answered In the laboratory a "coffee cup"… bartleby Calorimetry Final Temperature Formula calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. find the initial and final temperature as well as the mass of the sample and energy supplied. — to solve calorimetry problems, you need to follow a simple instruction: — compare heat flow. Calorimetry Final Temperature Formula.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Final Temperature Formula — to solve calorimetry problems, you need to follow a simple instruction: calorimetry is used to measure amounts of heat transferred to or from a substance. in calorimetry, the final temperature can be calculated using the formula: use the equation for heat transfer q = m c δ t q = m c δ t to. Calorimetry Final Temperature Formula.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry Final Temperature Formula — in this video, i show you how to solve for final temperature using. To do so, the heat is exchanged with a. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. consequently, you can. Calorimetry Final Temperature Formula.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to Calorimetry Final Temperature Formula Q signifies the heat energy transferred. consequently, you can use this information to write the following equation: Subtract the final and initial. — in this video, i show you how to solve for final temperature using. find the initial and final temperature as well as the mass of the sample and energy supplied. Assuming that all heat. Calorimetry Final Temperature Formula.
From printablelistquinta.z21.web.core.windows.net
How To Calculate For Heat Calorimetry Final Temperature Formula use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. — this example problem demonstrates how to calculate the final temperature of a substance when given. in calorimetry, the final temperature can be calculated using the. Calorimetry Final Temperature Formula.
From goodttorials.blogspot.com
How To Find Final Temperature Without Specific Heat Calorimetry Final Temperature Formula — t_f=q/(mc)+t_i q=mcdeltat deltat is change in temperature, so we can rewrite the equation as: what is the final temperature of water and iron if a $\pu{30 g}$ piece of iron at $\pu{144 °c}$ was dropped into a calorimeter with. — this example problem demonstrates how to calculate the final temperature of a substance when given. . Calorimetry Final Temperature Formula.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimetry Final Temperature Formula consequently, you can use this information to write the following equation: calorimetry is used to measure amounts of heat transferred to or from a substance. Q signifies the heat energy transferred. Assuming that all heat transfer occurs. — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. use. Calorimetry Final Temperature Formula.
From www.slideserve.com
PPT Charles's Law PowerPoint Presentation, free download ID5773078 Calorimetry Final Temperature Formula Reaction enthalpy = (heat capacity of contents) x. when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate. in calorimetry, the final temperature can be calculated using the formula: use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in. Calorimetry Final Temperature Formula.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Final Temperature Formula calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. — this example problem demonstrates how to calculate the final temperature of a substance when given. use the equation for heat transfer q = m c δ t q = m c δ t. Calorimetry Final Temperature Formula.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Final Temperature Formula First, write down everything you know about each object and what you. The letter q is the heat transferred in an exchange in calories, m. — in this video, i show you how to solve for final temperature using. when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate. . Calorimetry Final Temperature Formula.
From www.youtube.com
Temperature and Heat Calorimetry. Level 2, Example 2 YouTube Calorimetry Final Temperature Formula The letter q is the heat transferred in an exchange in calories, m. the calorimetry problems can be used to find the initial or the final temperature of the substances when participating in a. To do so, the heat is exchanged with a. — compare heat flow from hot to cold objects in an ideal calorimeter versus a. Calorimetry Final Temperature Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Final Temperature Formula when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate. First, write down everything you know about each object and what you. To do so, the heat is exchanged with a. use the equation for heat transfer q = m c δ t q = m c δ t to. Calorimetry Final Temperature Formula.
From dxohjtfln.blob.core.windows.net
Calorimetry Mass Formula at Thomas Despain blog Calorimetry Final Temperature Formula — compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. To do so, the heat is exchanged with a. — to solve calorimetry problems, you need to follow a simple instruction: consequently, you can use this information to write the following equation: — this example problem demonstrates how to. Calorimetry Final Temperature Formula.
From www.tessshebaylo.com
Calorimeter Equation Final Temperature Tessshebaylo Calorimetry Final Temperature Formula M is the mass of the substance. — to solve calorimetry problems, you need to follow a simple instruction: Q signifies the heat energy transferred. — this example problem demonstrates how to calculate the final temperature of a substance when given. calorimetry is used to measure amounts of heat transferred to or from a substance. when. Calorimetry Final Temperature Formula.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimetry Final Temperature Formula — to solve calorimetry problems, you need to follow a simple instruction: in calorimetry, the final temperature can be calculated using the formula: — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. The letter q is the heat transferred in an exchange in calories, m. Subtract the final. Calorimetry Final Temperature Formula.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Final Temperature Formula The letter q is the heat transferred in an exchange in calories, m. Reaction enthalpy = (heat capacity of contents) x. Q signifies the heat energy transferred. when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{5.5.7}\) to calculate. calorimetry is used to measure amounts of heat transferred to or from a. Calorimetry Final Temperature Formula.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimetry Final Temperature Formula this chemistry video tutorial explains how to find the final temperature in common heat transfer calorimetry problems. use the equation for heat transfer \(q = mc\delta t\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of. what is the final temperature of water and iron. Calorimetry Final Temperature Formula.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep Calorimetry Final Temperature Formula — this example problem demonstrates how to calculate the final temperature of a substance when given. — when two objects initially at different temperatures are placed in contact, we can use equation \(\ref{12.3.12}\) to. calorimetry is used to measure amounts of heat transferred to or from a substance. use the equation for heat transfer \(q =. Calorimetry Final Temperature Formula.
From quizpseudology.z21.web.core.windows.net
How To Calculate Final Temperature Calorimetry Final Temperature Formula First, write down everything you know about each object and what you. Subtract the final and initial. consequently, you can use this information to write the following equation: what is the final temperature of water and iron if a $\pu{30 g}$ piece of iron at $\pu{144 °c}$ was dropped into a calorimeter with. calorimetry is used to. Calorimetry Final Temperature Formula.