Lead Carbonate G/Mol . C 2 h 2 o 8 pb 3. molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. lead (ii) carbonate msds (material safety data sheet) or sds, coa and coq, dossiers, brochures and other available documents. this monograph for lead carbonate provides, in addition to common physical constants, a general description. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. Grams = mole × molar mass. Write down the chemical formula of the compound. herein, molecular dynamics simulations are employed to examine the chemical properties of li + when. lead carbonate forms colorless orthorhombic crystals; steps to calculate molar mass. lead carbonate other names: lead (ii) carbonate is used in polymerization catalysts. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: Pb (co 3) 2 molar mass conversion. It decomposes at about 315 °c.
from slidetodoc.com
Pb (co 3) 2 molar mass conversion. It decomposes at about 315 °c. lead carbonate is one of the major forms of lead present in freshwater. lead (ii) carbonate is used in polymerization catalysts. Data at other public nist sites: this monograph for lead carbonate provides, in addition to common physical constants, a general description. C 2 h 2 o 8 pb 3. lead carbonate other names: Mole is a standard scientific unit. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass:
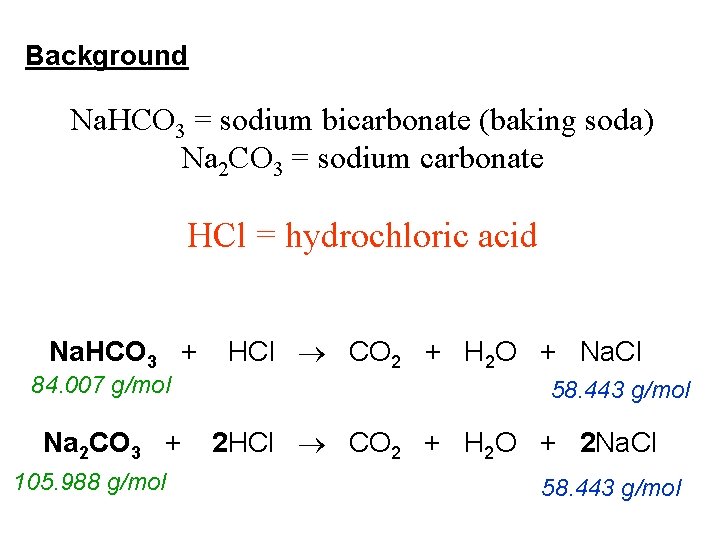
Lab 8 Sodium Carbonate or Sodium Bicarbonate Objective
Lead Carbonate G/Mol molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. lead carbonate other names: Mole is a standard scientific unit. this monograph for lead carbonate provides, in addition to common physical constants, a general description. Pb (co 3) 2 molar mass converter. For example, water is h. lead carbonate is one of the major forms of lead present in freshwater. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: Data at other public nist sites: It decomposes at about 315 °c. the first step to finding the molar mass of lead carbonate is to count the number of each atom present in a single molecule. Grams = mole × molar mass. steps to calculate molar mass. lead (ii) carbonate msds (material safety data sheet) or sds, coa and coq, dossiers, brochures and other available documents. C 2 h 2 o 8 pb 3. lead (ii) carbonate is used in polymerization catalysts.
From www.coursehero.com
[Solved] 100 mL of 0.2 mol/L sodium carbonate solution and 200 mL of 0. Lead Carbonate G/Mol calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. Pb (co 3) 2 molar mass converter. herein, molecular dynamics simulations are employed to examine the chemical properties of li + when. Pb (co 3) 2 molar mass conversion. Mole is a standard scientific unit. steps to calculate. Lead Carbonate G/Mol.
From www.shouyiresearch.com
346.38 G/Mol Chemical Raw Materials Diglycol Dimaleate Mild Odor Lead Carbonate G/Mol lead (ii) carbonate is used in polymerization catalysts. lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate lead iodide; calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. It decomposes at. Lead Carbonate G/Mol.
From www.youtube.com
Mass of Ca in 24.5 g sample of calcium carbonate YouTube Lead Carbonate G/Mol the first step to finding the molar mass of lead carbonate is to count the number of each atom present in a single molecule. Its concentration is limited due to its low. herein, molecular dynamics simulations are employed to examine the chemical properties of li + when. Grams = mole × molar mass. Pb (co 3) 2 molar. Lead Carbonate G/Mol.
From www.tradeindia.com
825 Degree C Melting 100.0869 G/mol Calcium Carbonate For Industrial Lead Carbonate G/Mol Mole is a standard scientific unit. Pb (co 3) 2 molar mass conversion. Write down the chemical formula of the compound. lead (ii) carbonate msds (material safety data sheet) or sds, coa and coq, dossiers, brochures and other available documents. It decomposes at about 315 °c. lead carbonate other names: Its concentration is limited due to its low.. Lead Carbonate G/Mol.
From www2.mdpi.com
Minerals Free FullText Pb Mineral Precipitation in Solutions of Lead Carbonate G/Mol Its concentration is limited due to its low. Mole is a standard scientific unit. this monograph for lead carbonate provides, in addition to common physical constants, a general description. lead (ii) carbonate is used in polymerization catalysts. lead carbonate is one of the major forms of lead present in freshwater. lead (ii) carbonate msds (material safety. Lead Carbonate G/Mol.
From askfilo.com
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 accordi.. Lead Carbonate G/Mol For example, water is h. lead carbonate forms colorless orthorhombic crystals; Write down the chemical formula of the compound. lead carbonate is one of the major forms of lead present in freshwater. Pb (co 3) 2 molar mass conversion. Data at other public nist sites: Mole is a standard scientific unit. lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate. Lead Carbonate G/Mol.
From www.numerade.com
SOLVED Consider the of calcium carbonate CaCO3 (s Lead Carbonate G/Mol lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate lead iodide; For example, water is h. Its concentration is limited due to its low. Pb (co 3) 2 molar mass converter. Data at other public nist sites: calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. Pb (co 3) 2 molar. Lead Carbonate G/Mol.
From chart-studio.plotly.com
Hydrochloric Acid (HCL) vs Calcium Carbonate (CaCO3) scatter chart Lead Carbonate G/Mol lead carbonate other names: Grams = mole × molar mass. the first step to finding the molar mass of lead carbonate is to count the number of each atom present in a single molecule. lead carbonate is one of the major forms of lead present in freshwater. Mole is a standard scientific unit. Pb (co 3) 2. Lead Carbonate G/Mol.
From www.youtube.com
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 Lead Carbonate G/Mol lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate lead iodide; lead (ii) carbonate is used in polymerization catalysts. molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: C 2 h 2. Lead Carbonate G/Mol.
From socratic.org
How many moles of carbon dioxide are produced when "50 g" of calcium Lead Carbonate G/Mol molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. this monograph for lead carbonate provides, in addition to common physical constants, a general description. For example, water is h. lead (ii) carbonate msds (material safety data sheet) or sds, coa and coq, dossiers, brochures and other available documents.. Lead Carbonate G/Mol.
From www.numerade.com
SOLVED According to the following reaction, how many moles of Lead Carbonate G/Mol Data at other public nist sites: this monograph for lead carbonate provides, in addition to common physical constants, a general description. C 2 h 2 o 8 pb 3. Its concentration is limited due to its low. lead carbonate is one of the major forms of lead present in freshwater. lead carbonate forms colorless orthorhombic crystals; . Lead Carbonate G/Mol.
From www.grainger.com
598630, F.W. 267.20, Lead Carbonate, Powder, Reagent, ACS 39G579 Lead Carbonate G/Mol this monograph for lead carbonate provides, in addition to common physical constants, a general description. lead carbonate is one of the major forms of lead present in freshwater. Its concentration is limited due to its low. molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. lead carbonate. Lead Carbonate G/Mol.
From www.coursehero.com
[Solved] Volume sodium carbonate (ml) 96.0 Molarity sodium carbonate (M Lead Carbonate G/Mol lead carbonate is one of the major forms of lead present in freshwater. Data at other public nist sites: C 2 h 2 o 8 pb 3. Grams = mole × molar mass. It decomposes at about 315 °c. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance.. Lead Carbonate G/Mol.
From www.kenyaplex.com
In an experiment to determine the percentage purity of the sample of Lead Carbonate G/Mol calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. Grams = mole × molar mass. steps to calculate molar mass. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. this monograph for lead carbonate provides, in. Lead Carbonate G/Mol.
From www.numerade.com
SOLVED A 6.53 g sample of a mixture of magnesium carbonate and Lead Carbonate G/Mol Pb (co 3) 2 molar mass conversion. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. C 2 h 2 o 8 pb 3. Its concentration is limited due to its low. the first step to finding the molar mass of lead carbonate is to count the number. Lead Carbonate G/Mol.
From lessonlibraryvamplate.z14.web.core.windows.net
Gram To Gram Conversion Calculator Chemistry Lead Carbonate G/Mol lead (ii) carbonate is used in polymerization catalysts. For example, water is h. lead carbonate forms colorless orthorhombic crystals; C 2 h 2 o 8 pb 3. Grams = mole × molar mass. Pb (co 3) 2 molar mass converter. herein, molecular dynamics simulations are employed to examine the chemical properties of li + when. It decomposes. Lead Carbonate G/Mol.
From savanahminwells.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid SavanahminWells Lead Carbonate G/Mol Grams = mole × molar mass. For example, water is h. C 2 h 2 o 8 pb 3. C 2 h 2 o 8 pb 3. lead carbonate is one of the major forms of lead present in freshwater. lead (ii) carbonate msds (material safety data sheet) or sds, coa and coq, dossiers, brochures and other available. Lead Carbonate G/Mol.
From www.fishersci.es
Carbonato de litio, 99,99 (base de metales sin Ca), Thermo Scientific Lead Carbonate G/Mol Its concentration is limited due to its low. steps to calculate molar mass. Pb (co 3) 2 molar mass conversion. It decomposes at about 315 °c. For example, water is h. lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate lead iodide; lead carbonate other names: calculate the molar mass of lead(ii) carbonate in grams per mole or search. Lead Carbonate G/Mol.
From www.chegg.com
Solved Consider the of calcium carbonate Lead Carbonate G/Mol calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. For example, water is h. lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate lead iodide; lead carbonate is one of the major forms of lead present in freshwater. Write down the chemical formula of the compound. lead (ii) carbonate. Lead Carbonate G/Mol.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Lead Carbonate G/Mol steps to calculate molar mass. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. Data at other public nist sites: this monograph for lead carbonate provides, in addition to common physical constants, a general description. C 2 h 2 o 8 pb 3. For example, water is. Lead Carbonate G/Mol.
From byjus.com
77. 25 ml of a solution containing NaOH and Na2CO3(sodium bi carbonate Lead Carbonate G/Mol this monograph for lead carbonate provides, in addition to common physical constants, a general description. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: Pb (co 3) 2 molar. Lead Carbonate G/Mol.
From w20.b2m.cz
Um Quimico Preparou Uma Solução De Carbonato EDUCA Lead Carbonate G/Mol the first step to finding the molar mass of lead carbonate is to count the number of each atom present in a single molecule. steps to calculate molar mass. lead (ii) carbonate is used in polymerization catalysts. Grams = mole × molar mass. lead (ii) carbonate msds (material safety data sheet) or sds, coa and coq,. Lead Carbonate G/Mol.
From www.youtube.com
Molar Mass / Molecular Weight of Pb(CO3)2 Lead (IV) carbonate YouTube Lead Carbonate G/Mol the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: Pb (co 3) 2 molar mass conversion. herein, molecular dynamics simulations are employed to examine the chemical properties of li + when. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula. Lead Carbonate G/Mol.
From slideplayer.com
Determining Chemical Formulas ppt download Lead Carbonate G/Mol lead (ii) carbonate is used in polymerization catalysts. steps to calculate molar mass. lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate lead iodide; For example, water is h. Write down the chemical formula of the compound. Its concentration is limited due to its low. Mole is a standard scientific unit. lead carbonate other names: calculate the molar. Lead Carbonate G/Mol.
From www.grainger.com
598630, F.W. 267.20, Lead Carbonate, Powder, Reagent, ACS 39G576 Lead Carbonate G/Mol lead (ii) carbonate msds (material safety data sheet) or sds, coa and coq, dossiers, brochures and other available documents. lead carbonate is one of the major forms of lead present in freshwater. Data at other public nist sites: steps to calculate molar mass. lead carbonate forms colorless orthorhombic crystals; lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate. Lead Carbonate G/Mol.
From slideplayer.com
The Mole Molar Conversions. ppt download Lead Carbonate G/Mol this monograph for lead carbonate provides, in addition to common physical constants, a general description. Mole is a standard scientific unit. lead (ii) carbonate msds (material safety data sheet) or sds, coa and coq, dossiers, brochures and other available documents. Its concentration is limited due to its low. Write down the chemical formula of the compound. Pb (co. Lead Carbonate G/Mol.
From www.fishersci.es
Carbonato de plomo(II), reactivo ACS, ACROS Organics™ Otros compuestos Lead Carbonate G/Mol the first step to finding the molar mass of lead carbonate is to count the number of each atom present in a single molecule. lead carbonate is one of the major forms of lead present in freshwater. Write down the chemical formula of the compound. For example, water is h. lead (ii) carbonate is used in polymerization. Lead Carbonate G/Mol.
From www.quanswer.com
Chemical analysis shows that a compound with a molar mass of 58g/mol Lead Carbonate G/Mol molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. Mole is a standard scientific unit. lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate lead iodide; the mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: C 2 h 2 o 8 pb. Lead Carbonate G/Mol.
From steventaroperkins.blogspot.com
g/mol to kg/kmol SteventaroPerkins Lead Carbonate G/Mol calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. lead (ii) carbonate is used in polymerization catalysts. Write down the chemical formula of the compound. lead carbonate other names: C 2 h 2 o 8 pb 3. steps to calculate molar mass. For example, water is. Lead Carbonate G/Mol.
From www.youtube.com
Calculating Molarity, Solving for Moles & Grams, 4 Practice Examples Lead Carbonate G/Mol molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. Write down the chemical formula of the compound. steps to calculate molar mass. lead (ii) carbonate is used in polymerization catalysts. herein, molecular dynamics simulations are employed to examine the chemical properties of li + when. Grams =. Lead Carbonate G/Mol.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Lead Carbonate G/Mol Mole is a standard scientific unit. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. C 2 h 2 o 8 pb 3. steps to calculate molar mass. C 2 h 2 o 8 pb 3. Write down the chemical formula of the compound. calculate the molar. Lead Carbonate G/Mol.
From www.researchgate.net
ESI mass spectrum of oligo(propylene carbonate) of Mn ≈900 g/mol Lead Carbonate G/Mol Mole is a standard scientific unit. Grams = mole × molar mass. lead carbonate other names: lead (ii) carbonate is used in polymerization catalysts. Write down the chemical formula of the compound. the first step to finding the molar mass of lead carbonate is to count the number of each atom present in a single molecule. Pb. Lead Carbonate G/Mol.
From www.coursehero.com
[Solved] A 0.849 g sample containing Ag2O (MM = 231.7358 g/mol) is Lead Carbonate G/Mol lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate lead iodide; Pb (co 3) 2 molar mass converter. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. steps to calculate. Lead Carbonate G/Mol.
From slidetodoc.com
Lab 8 Sodium Carbonate or Sodium Bicarbonate Objective Lead Carbonate G/Mol steps to calculate molar mass. lead (ii) carbonate is used in polymerization catalysts. C 2 h 2 o 8 pb 3. Data at other public nist sites: Write down the chemical formula of the compound. Grams = mole × molar mass. molar mass (molar weight) is the mass of one mole of a substance and is expressed. Lead Carbonate G/Mol.
From solvedlib.com
How many moles of sodium carbonate are equired to pre… SolvedLib Lead Carbonate G/Mol lead(ii) chloride lead(ii) chromate lead(ii) tetrafluoroborate lead iodide; Data at other public nist sites: C 2 h 2 o 8 pb 3. calculate the molar mass of lead(ii) carbonate in grams per mole or search for a chemical formula or substance. lead carbonate other names: this monograph for lead carbonate provides, in addition to common physical. Lead Carbonate G/Mol.