Resorcinol And Hydroquinone . selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of. It is an organic compound with the formula c 6 h 4 (oh) 2. a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made to. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene, which have been. to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. a quick search on says that resorcinol is more acidic that catechol, followed by phenol. it is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. resorcinol (or resorcin) is a phenolic compound.
from www.semanticscholar.org
it is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene, which have been. selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. It is an organic compound with the formula c 6 h 4 (oh) 2. sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of. the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various.
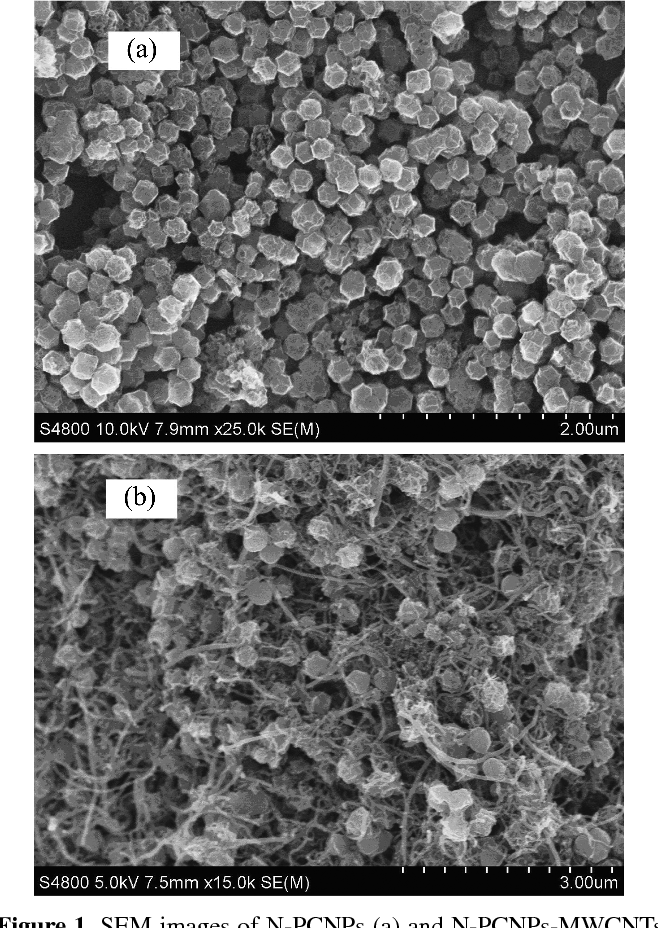
Figure 1 from Simultaneous Electrochemical Determination of
Resorcinol And Hydroquinone the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. it is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made to. Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). resorcinol (or resorcin) is a phenolic compound. catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene, which have been. the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. a quick search on says that resorcinol is more acidic that catechol, followed by phenol. sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. It is an organic compound with the formula c 6 h 4 (oh) 2. using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the.
From www.researchgate.net
Competitive reaction of catechol and resorcinol. Conditions 323 K, 3 Resorcinol And Hydroquinone catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene, which have been. Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. it is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. the present study reviews the adsorptive removal of catechol (c), resorcinol. Resorcinol And Hydroquinone.
From benthamopen.com
Poly (Adenine) Modified GrapheneBased Voltammetric Sensor for the Resorcinol And Hydroquinone a quick search on says that resorcinol is more acidic that catechol, followed by phenol. a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made to. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05. Resorcinol And Hydroquinone.
From www.youtube.com
Detection of Hydroquinone, Catechol and Resorcinol YouTube Resorcinol And Hydroquinone it is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. resorcinol (or resorcin) is a phenolic compound. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made. Resorcinol And Hydroquinone.
From pubs.rsc.org
Isomerdependent catalytic pyrolysis mechanism of the lignin model Resorcinol And Hydroquinone the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. It is an organic compound with the formula c 6 h 4 (oh) 2. a quick search on says that resorcinol is more acidic that catechol, followed by phenol. . Resorcinol And Hydroquinone.
From www.researchgate.net
Simultaneous determination of hydroquinone (HQ) and catechol (CC) in Resorcinol And Hydroquinone selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. It is an organic compound with the formula c 6 h. Resorcinol And Hydroquinone.
From stock.adobe.com
chemical structure of types of phenols. Phenol catechol resorcinol Resorcinol And Hydroquinone to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). sensitive and facile determination of catechol and hydroquinone simultaneously under. Resorcinol And Hydroquinone.
From www.researchgate.net
(PDF) Competitive Hydrogenation and Hydrodeoxygenation of Oxygen Resorcinol And Hydroquinone sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of. to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was. Resorcinol And Hydroquinone.
From pubs.rsc.org
Electrochemical measurements and theoretical studies for understanding Resorcinol And Hydroquinone using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made to. It is an organic compound with the formula c. Resorcinol And Hydroquinone.
From www.jennyskincare.com
PCA SKIN PCA Peel with Hydroquinone & Resorcinol 0.25oz / 7.4ml Resorcinol And Hydroquinone a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made to. catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene, which have been. the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. it is used as an. Resorcinol And Hydroquinone.
From www.semanticscholar.org
Figure 1 from Simultaneous Electrochemical Determination of Resorcinol And Hydroquinone It is an organic compound with the formula c 6 h 4 (oh) 2. resorcinol (or resorcin) is a phenolic compound. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. using electrochemical techniques (cyclic voltammetry (cv) and differential. Resorcinol And Hydroquinone.
From www.suddenlyslimmer.com
PCA Hydroquinone & Resorcinol Peel in Phoenix & Scottsdale, AZ Resorcinol And Hydroquinone Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). It is an organic compound with the formula c 6 h. Resorcinol And Hydroquinone.
From pubs.rsc.org
Isomerdependent catalytic pyrolysis mechanism of the lignin model Resorcinol And Hydroquinone using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. resorcinol (or resorcin) is a phenolic compound. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of. the present study reviews the adsorptive removal of catechol (c), resorcinol. Resorcinol And Hydroquinone.
From byjus.com
boiling point order of phenol,catechol,resorcinol,quinole Resorcinol And Hydroquinone selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. it is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. resorcinol (or resorcin). Resorcinol And Hydroquinone.
From www.researchgate.net
(PDF) Adsorption of catechol, resorcinol, hydroquinone, and their Resorcinol And Hydroquinone sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of. the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. it is used as an antiseptic and disinfectant in topical pharmaceutical products in. Resorcinol And Hydroquinone.
From pubs.rsc.org
Isomerdependent catalytic pyrolysis mechanism of the lignin model Resorcinol And Hydroquinone catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene, which have been. a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made to. to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. using electrochemical techniques (cyclic voltammetry (cv) and differential. Resorcinol And Hydroquinone.
From www.alamyimages.fr
Le catéchol, résorcinol , molécule hydroquinone Structure de formule Resorcinol And Hydroquinone resorcinol (or resorcin) is a phenolic compound. Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. the present study reviews the adsorptive removal of. Resorcinol And Hydroquinone.
From www.researchgate.net
(PDF) Agomelatine cocrystals with resorcinol and hydroquinone Resorcinol And Hydroquinone to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. a quick search on says that resorcinol is more acidic that catechol, followed by phenol. catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene,. Resorcinol And Hydroquinone.
From www.researchgate.net
1 H NMR spectra of phenol, hydroquinone and resorcinol (20 mM each) in Resorcinol And Hydroquinone selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). It is an organic compound with the formula c 6 h 4 (oh) 2. a review of the published industrial toxicology for the. Resorcinol And Hydroquinone.
From www.fishersci.es
Resorcinol, extrapuro, SLR, Fisher Chemical Fisher Scientific Resorcinol And Hydroquinone it is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. It is an organic compound with the formula c 6 h 4 (oh) 2. the present study reviews the adsorptive removal of catechol (c),. Resorcinol And Hydroquinone.
From www.researchgate.net
Design of tyrosinase inhibitors A, hydroquinone; B, resorcinol; C Resorcinol And Hydroquinone catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. a quick search on says that resorcinol is more acidic that catechol, followed by phenol. using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. sensitive and facile determination of. Resorcinol And Hydroquinone.
From studylib.net
The Dipole Moments of Catechol, Resorcinol and Hydroquinone` Resorcinol And Hydroquinone selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. a quick search on says that resorcinol is more acidic that. Resorcinol And Hydroquinone.
From dokumen.tips
(PDF) catechol, resorcinol, hydroquinone DOKUMEN.TIPS Resorcinol And Hydroquinone the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. resorcinol (or resorcin) is a phenolic compound.. Resorcinol And Hydroquinone.
From pleijsalon.com
Jessner Peel Treatments What to Know PLEIJ Salon + Spa Resorcinol And Hydroquinone Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with. Resorcinol And Hydroquinone.
From www.researchgate.net
(PDF) Competitive Hydrogenation and Hydrodeoxygenation of Oxygen Resorcinol And Hydroquinone to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. it is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula c 6 h 4 (oh) 2.. Resorcinol And Hydroquinone.
From www.researchgate.net
(PDF) Voltammetric approach for pharmaceutical samples analysis Resorcinol And Hydroquinone using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). it is used as an antiseptic and disinfectant in topical pharmaceutical products in the. Resorcinol And Hydroquinone.
From www.researchgate.net
Experimental solubility of salicylic acid, resorcinol, hydroquinone in Resorcinol And Hydroquinone using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene, which have been. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). resorcinol (or resorcin) is a phenolic compound. sensitive and facile determination. Resorcinol And Hydroquinone.
From www.semanticscholar.org
Figure 2 from Simultaneous and selective electrochemical determination Resorcinol And Hydroquinone it is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene, which have been. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). to develop sustainable lignin. Resorcinol And Hydroquinone.
From www.studocu.com
Lec 2 Biochemistry phenol OH OH OH hydroquinone (quinol Resorcinol And Hydroquinone sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of. a quick search on says that resorcinol is more acidic that catechol, followed by phenol. to develop sustainable lignin valorization strategies, a solid understanding of the underlying reaction mechanism is critical. resorcinol (or resorcin) is a phenolic compound. catechol (cc), resorcinol (rs), and. Resorcinol And Hydroquinone.
From www.researchgate.net
(PDF) Simultaneous Determination of Hydroquinone, Catechol and Resorcinol And Hydroquinone selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. sensitive and facile determination of catechol and hydroquinone simultaneously under coexistence of. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which are. the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium. Resorcinol And Hydroquinone.
From www.renu21usa.com
PCA Professional MediumDepth Chemical Peels At RENU21 Facial Spa Resorcinol And Hydroquinone a quick search on says that resorcinol is more acidic that catechol, followed by phenol. a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made to. using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. catechol (ct), resorcinol (rs) and hydroquinone (hq) are three dihydroxybenzene isomers, which. Resorcinol And Hydroquinone.
From www.youtube.com
PCA Peel + Hydroquinone And Resorcinol Application YouTube Resorcinol And Hydroquinone a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made to. using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula c 6 h 4 (oh) 2. catechol (ct), resorcinol (rs) and hydroquinone. Resorcinol And Hydroquinone.
From www.openpr.com
Dihydroxybenzenes (Catechol, Resorcinol, Hydroquinone) Resorcinol And Hydroquinone a quick search on says that resorcinol is more acidic that catechol, followed by phenol. a review of the published industrial toxicology for the benzenediols, catechol, resorcinol and hydroquinone was made to. the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. the nitration and nitrosation reactions. Resorcinol And Hydroquinone.
From pubs.rsc.org
Spectrophotometric determination of microgram amounts of hydroquinone Resorcinol And Hydroquinone the present study reviews the adsorptive removal of catechol (c), resorcinol (r), hydroquinone (hq), and their derivatives from various. resorcinol (or resorcin) is a phenolic compound. Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. using electrochemical. Resorcinol And Hydroquinone.
From www.sarthaks.com
Which of the following is the wekest acid? Sarthaks eConnect Resorcinol And Hydroquinone the nitration and nitrosation reactions of catechol, resorcinol, and hydroquinone (0.05 mmol/l) with sodium nitrite (0.05 mmol/l). Worldwide manufacturing capacities of hydroquinone, resorcinol, and catechol are 50,000, 30,000,. resorcinol (or resorcin) is a phenolic compound. selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. sensitive and facile determination. Resorcinol And Hydroquinone.
From www.researchgate.net
(PDF) Acid‐ and base‐catalyzed epoxy‐phenol thermosets from catechol Resorcinol And Hydroquinone resorcinol (or resorcin) is a phenolic compound. catechol (cc), resorcinol (rs), and hydroquinone (hq) are three isomers of dihydroxybenzene, which have been. selective processes leading to hydroquinone are achieved by the oxidation of aniline using manganese dioxide or the. using electrochemical techniques (cyclic voltammetry (cv) and differential pulse voltammetry (dpv)) with. it is used as. Resorcinol And Hydroquinone.