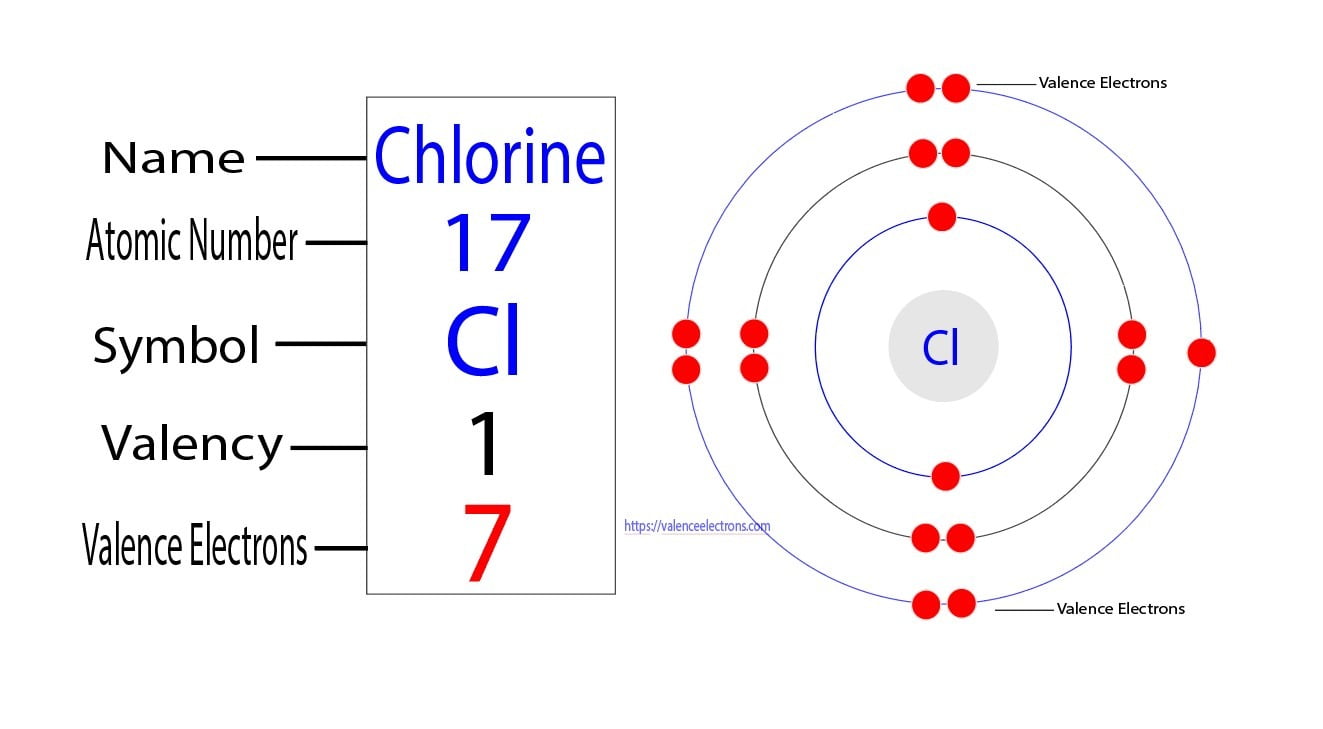
Does Chlorine Have A Larger Atomic Radius Than Phosphorus . This table shows how the atom size, and atomic radius values change. The graph shows how atomic radius varies across period 3: The atomic radius of a chemical element is a measure of the. Atom size values are calculated from atomic radius data. In both cases, there is a periodic table trend. Chlorine, cl 2, is a much smaller molecule. Each atom’s size is relative to the largest element, cesium. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. As the atomic number increases, the atomic radius decreases. Below mentioned radii are the van der waals radius in. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in,. Atomic radius of all the elements are mentioned in the chart below. The atomic radius of chlorine atom is 102pm (covalent radius). The size of atoms of elements may be expressed in terms of atomic radius or ionic radius.
from loeylrncp.blob.core.windows.net
Each atom’s size is relative to the largest element, cesium. The atomic radius of a chemical element is a measure of the. Chlorine, cl 2, is a much smaller molecule. Atom size values are calculated from atomic radius data. This table shows how the atom size, and atomic radius values change. The atomic radius of chlorine atom is 102pm (covalent radius). The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. In both cases, there is a periodic table trend. Atomic radius of all the elements are mentioned in the chart below. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius.
Chlorine Electrons And Protons at Edward Thompson blog
Does Chlorine Have A Larger Atomic Radius Than Phosphorus This table shows how the atom size, and atomic radius values change. As the atomic number increases, the atomic radius decreases. The graph shows how atomic radius varies across period 3: This table shows how the atom size, and atomic radius values change. The atomic radius of a chemical element is a measure of the. Below mentioned radii are the van der waals radius in. In both cases, there is a periodic table trend. Each atom’s size is relative to the largest element, cesium. The atomic radius of chlorine atom is 102pm (covalent radius). Atom size values are calculated from atomic radius data. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in,. Chlorine, cl 2, is a much smaller molecule. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. Atomic radius of all the elements are mentioned in the chart below.
From elchoroukhost.net
Largest Atomic Radius Periodic Table Elcho Table Does Chlorine Have A Larger Atomic Radius Than Phosphorus Atomic radius of all the elements are mentioned in the chart below. As the atomic number increases, the atomic radius decreases. Atom size values are calculated from atomic radius data. The graph shows how atomic radius varies across period 3: The atomic radius of a chemical element is a measure of the. The atomic radius of chlorine atom is 102pm. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From brainly.in
Draw the atomic structure of a chlorine ion Brainly.in Does Chlorine Have A Larger Atomic Radius Than Phosphorus In both cases, there is a periodic table trend. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in,. Each atom’s size is relative to the largest element, cesium. Atomic radius of. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From commons.wikimedia.org
Original file (SVG file, nominally 851 × 520 pixels, file size 91 KB) Does Chlorine Have A Larger Atomic Radius Than Phosphorus An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in,. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. In both cases, there is. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From loeylrncp.blob.core.windows.net
Chlorine Electrons And Protons at Edward Thompson blog Does Chlorine Have A Larger Atomic Radius Than Phosphorus Below mentioned radii are the van der waals radius in. The atomic radius of a chemical element is a measure of the. This table shows how the atom size, and atomic radius values change. As the atomic number increases, the atomic radius decreases. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius.. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From gia-kharmon.blogspot.com
What Is the Atomic Radius of Chlorine Does Chlorine Have A Larger Atomic Radius Than Phosphorus Chlorine, cl 2, is a much smaller molecule. The atomic radius of a chemical element is a measure of the. The atomic radius of chlorine atom is 102pm (covalent radius). The graph shows how atomic radius varies across period 3: As the atomic number increases, the atomic radius decreases. This table shows how the atom size, and atomic radius values. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From grademarkets.com
Understanding Atomic Radius Trends The 2 Key Principles Grade Markets Does Chlorine Have A Larger Atomic Radius Than Phosphorus The atomic radius of a chemical element is a measure of the. Each atom’s size is relative to the largest element, cesium. Chlorine, cl 2, is a much smaller molecule. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. As the atomic number increases,. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From animalia-life.club
Atomic Radius Diagram Does Chlorine Have A Larger Atomic Radius Than Phosphorus Atom size values are calculated from atomic radius data. Each atom’s size is relative to the largest element, cesium. Atomic radius of all the elements are mentioned in the chart below. Chlorine, cl 2, is a much smaller molecule. Below mentioned radii are the van der waals radius in. The molecules are bigger than phosphorus molecules, and so the van. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From www.studypool.com
SOLUTION Rank from highest to lowest atomic radiius, chemistry Does Chlorine Have A Larger Atomic Radius Than Phosphorus An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in,. In both cases, there is a periodic table trend. As the atomic number increases, the atomic radius decreases. Atom size values are. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From dokumen.tips
(PPTX) Review of Atomic Structure. Why does an ion of phosphorus, P 3 Does Chlorine Have A Larger Atomic Radius Than Phosphorus Below mentioned radii are the van der waals radius in. Atom size values are calculated from atomic radius data. Chlorine, cl 2, is a much smaller molecule. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. In both cases, there is a periodic table. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From mungfali.com
Atomic Radius Chart Periodic Table Does Chlorine Have A Larger Atomic Radius Than Phosphorus Each atom’s size is relative to the largest element, cesium. Below mentioned radii are the van der waals radius in. This table shows how the atom size, and atomic radius values change. The atomic radius of chlorine atom is 102pm (covalent radius). Atomic radius of all the elements are mentioned in the chart below. The molecules are bigger than phosphorus. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From www.animalia-life.club
Ionic Radius Diagram Does Chlorine Have A Larger Atomic Radius Than Phosphorus The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. As the atomic number increases, the atomic radius decreases. Atom size values are calculated from atomic radius data. Below mentioned radii are the van der waals radius in. Chlorine, cl 2, is a much smaller molecule. The molecules are bigger than phosphorus molecules,. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From www.ck12.org
Periodic Trends in Atomic Size CK12 Foundation Does Chlorine Have A Larger Atomic Radius Than Phosphorus Atomic radius of all the elements are mentioned in the chart below. The atomic radius of a chemical element is a measure of the. Each atom’s size is relative to the largest element, cesium. In both cases, there is a periodic table trend. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger,. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From learningzoneteiar09.z14.web.core.windows.net
Atomic Radius Trend Explained Does Chlorine Have A Larger Atomic Radius Than Phosphorus This table shows how the atom size, and atomic radius values change. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in,. The atomic radius of chlorine atom is 102pm (covalent radius).. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From www.coursehero.com
[Solved] 1. Explain why sulfur has a larger atomic radius than chlorine Does Chlorine Have A Larger Atomic Radius Than Phosphorus Atomic radius of all the elements are mentioned in the chart below. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in,. The atomic radius of a chemical element is a measure. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From material-properties.org
Phosphorus Periodic Table and Atomic Properties Does Chlorine Have A Larger Atomic Radius Than Phosphorus Atom size values are calculated from atomic radius data. Each atom’s size is relative to the largest element, cesium. In both cases, there is a periodic table trend. Chlorine, cl 2, is a much smaller molecule. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From sciencenotes.org
Atomic Radius and Ionic Radius Does Chlorine Have A Larger Atomic Radius Than Phosphorus Atom size values are calculated from atomic radius data. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. The graph shows how atomic radius varies across period. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From library.achievingthedream.org
Periodic Variations in Element Properties Introductory Chemistry Does Chlorine Have A Larger Atomic Radius Than Phosphorus As the atomic number increases, the atomic radius decreases. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. Each atom’s size is relative to the largest element, cesium. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From pediaa.com
Difference Between Atomic Radius and Ionic Radius Definition Does Chlorine Have A Larger Atomic Radius Than Phosphorus The graph shows how atomic radius varies across period 3: This table shows how the atom size, and atomic radius values change. The atomic radius of a chemical element is a measure of the. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From ecurrencythailand.com
Which Element Fluorine Or Chlorine Will Have The Greatest Atomic Radius Does Chlorine Have A Larger Atomic Radius Than Phosphorus As the atomic number increases, the atomic radius decreases. The atomic radius of chlorine atom is 102pm (covalent radius). Atom size values are calculated from atomic radius data. Each atom’s size is relative to the largest element, cesium. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. This table shows how the. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From courses.lumenlearning.com
Ionic Radius Introduction to Chemistry Does Chlorine Have A Larger Atomic Radius Than Phosphorus Atomic radius of all the elements are mentioned in the chart below. In both cases, there is a periodic table trend. The atomic radius of chlorine atom is 102pm (covalent radius). Each atom’s size is relative to the largest element, cesium. The atomic radius of a chemical element is a measure of the. The graph shows how atomic radius varies. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From www.numerade.com
SOLVED Question 4 (8 points) Saved Learning 4 Determine Does Chlorine Have A Larger Atomic Radius Than Phosphorus As the atomic number increases, the atomic radius decreases. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in,. Below mentioned radii are the van der waals radius in. Atomic radius of. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From www.pinterest.co.kr
The ionic radius of helium is 93. … Atom, Ionic radius, Teaching Does Chlorine Have A Larger Atomic Radius Than Phosphorus In both cases, there is a periodic table trend. The atomic radius of chlorine atom is 102pm (covalent radius). The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. Atom size values are calculated from atomic radius data. Below mentioned radii are the van der. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From dxorxejky.blob.core.windows.net
Does Chlorine Have A Larger Atomic Radius Than Fluorine at Leon Cain blog Does Chlorine Have A Larger Atomic Radius Than Phosphorus Chlorine, cl 2, is a much smaller molecule. This table shows how the atom size, and atomic radius values change. The atomic radius of a chemical element is a measure of the. Each atom’s size is relative to the largest element, cesium. Atomic radius of all the elements are mentioned in the chart below. As the atomic number increases, the. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From freyabartlett.z13.web.core.windows.net
Size Of Atoms Chart Does Chlorine Have A Larger Atomic Radius Than Phosphorus Below mentioned radii are the van der waals radius in. The atomic radius of a chemical element is a measure of the. The atomic radius of chlorine atom is 102pm (covalent radius). Chlorine, cl 2, is a much smaller molecule. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Does Chlorine Have A Larger Atomic Radius Than Phosphorus The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. This table shows how the atom size, and atomic radius values change. Atom size values are calculated from atomic radius data. Each atom’s size is relative to the largest element, cesium. An atom such as chlorine has both a covalent radius (the distance. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From www.numerade.com
SOLVED Sodium atoms are much larger than chlorine atoms, but sodium Does Chlorine Have A Larger Atomic Radius Than Phosphorus Atomic radius of all the elements are mentioned in the chart below. Atom size values are calculated from atomic radius data. In both cases, there is a periodic table trend. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From favpng.com
Atom Bohr Model Electron Configuration Chlorine, PNG, 1000x1000px, Atom Does Chlorine Have A Larger Atomic Radius Than Phosphorus This table shows how the atom size, and atomic radius values change. As the atomic number increases, the atomic radius decreases. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. The atomic radius of a chemical element is a measure of the. An atom such as chlorine has both a covalent radius. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Does Chlorine Have A Larger Atomic Radius Than Phosphorus As the atomic number increases, the atomic radius decreases. In both cases, there is a periodic table trend. Each atom’s size is relative to the largest element, cesium. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. The graph shows how atomic radius varies. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From eogaret.weebly.com
Which element has a larger atomic radius than sulfur eogaret Does Chlorine Have A Larger Atomic Radius Than Phosphorus This table shows how the atom size, and atomic radius values change. In both cases, there is a periodic table trend. Atomic radius of all the elements are mentioned in the chart below. As the atomic number increases, the atomic radius decreases. Chlorine, cl 2, is a much smaller molecule. Each atom’s size is relative to the largest element, cesium.. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From chemistry.stackexchange.com
What is the atomic radius of chlorine? Chemistry Stack Exchange Does Chlorine Have A Larger Atomic Radius Than Phosphorus Below mentioned radii are the van der waals radius in. The atomic radius of a chemical element is a measure of the. Chlorine, cl 2, is a much smaller molecule. An atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From mungfali.com
Largest Atomic Radius Periodic Table Periodic Table Timeline CDF Does Chlorine Have A Larger Atomic Radius Than Phosphorus The atomic radius of a chemical element is a measure of the. Atomic radius of all the elements are mentioned in the chart below. As the atomic number increases, the atomic radius decreases. The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. Each atom’s. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.
From sciencenotes.org
Atomic Radius and Ionic Radius Does Chlorine Have A Larger Atomic Radius Than Phosphorus The molecules are bigger than phosphorus molecules, and so the van der waals attractions will be stronger, leading to a higher melting and boiling point. The size of atoms of elements may be expressed in terms of atomic radius or ionic radius. This table shows how the atom size, and atomic radius values change. Each atom’s size is relative to. Does Chlorine Have A Larger Atomic Radius Than Phosphorus.