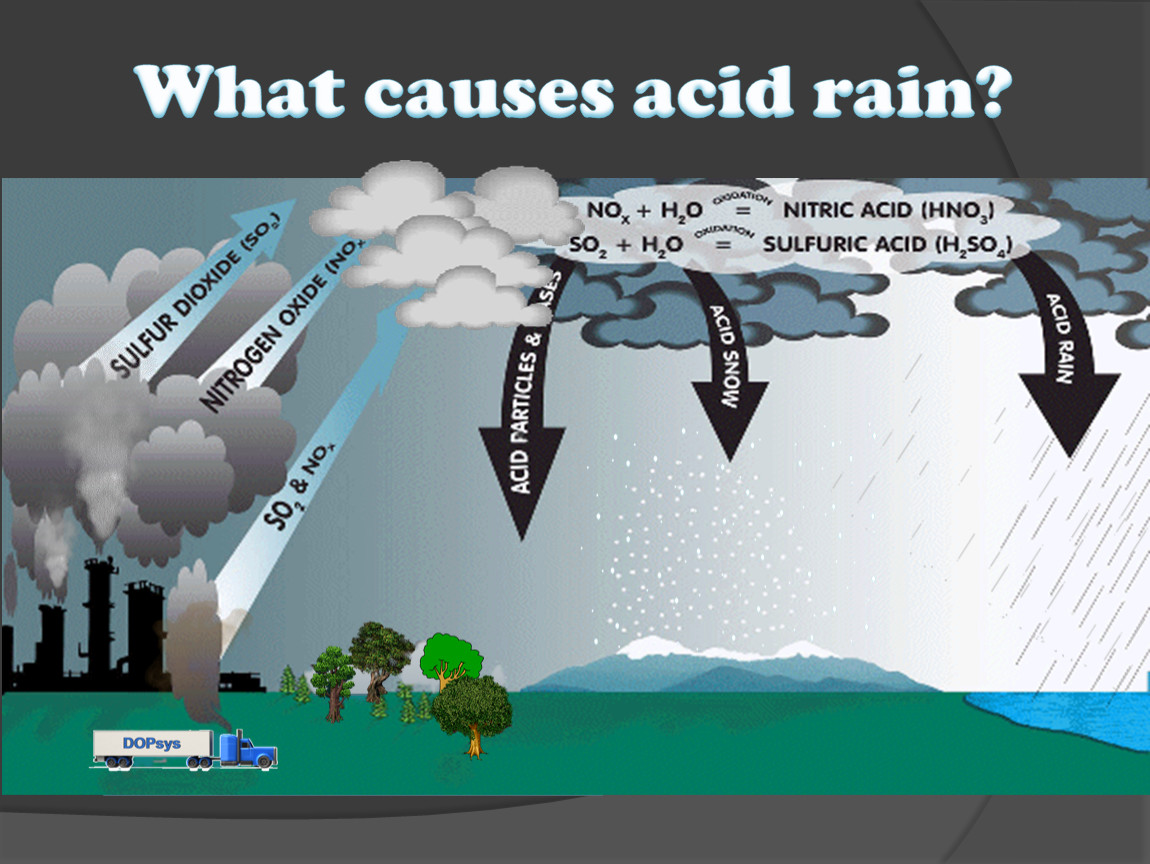
How Does Acid Rain Cause Damage . How does acid rain affect the environment? When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that make the particles acidic can land on statues, buildings, and. Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Acid rain nearly affects everything around us. When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. Dead or dying trees are a common sight in areas effected by acid rain. That aluminum may be harmful. Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm. Acid rain leaches aluminum from the soil. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2). Some significant consequences are listed below: Effects of acid rain on plants and trees. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a.
from znanio.ru
Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm. Acid rain leaches aluminum from the soil. When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. Effects of acid rain on plants and trees. Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2). Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to it containing dissolved sulphur dioxide or nitrogen oxides. How does acid rain affect the environment? Acid rain nearly affects everything around us.
Презентация по английскому языку для учащихся 10 класса "Acid Rain"
How Does Acid Rain Cause Damage That aluminum may be harmful. That aluminum may be harmful. Effects of acid rain on plants and trees. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that make the particles acidic can land on statues, buildings, and. Dead or dying trees are a common sight in areas effected by acid rain. Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Acid rain leaches aluminum from the soil. Some significant consequences are listed below: Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2). How does acid rain affect the environment? Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm. Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to it containing dissolved sulphur dioxide or nitrogen oxides.
From en.meteorologiaenred.com
The Venus How Does Acid Rain Cause Damage Some significant consequences are listed below: It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. That aluminum may be harmful. Acid rain leaches aluminum from the soil. Acid rain, precipitation possessing a. How Does Acid Rain Cause Damage.
From mungfali.com
Acid Rain Water Pollution How Does Acid Rain Cause Damage Acid rain leaches aluminum from the soil. Effects of acid rain on plants and trees. When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. Acid rain nearly affects everything around us. That. How Does Acid Rain Cause Damage.
From www.gardeningknowhow.com
Acid Rain And Plant Damage Effects Of Acid Rain On Plant Growth How Does Acid Rain Cause Damage How does acid rain affect the environment? Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to it containing dissolved sulphur dioxide or nitrogen oxides. Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors.. How Does Acid Rain Cause Damage.
From www.britannica.com
Acid Rain Saving Earth Encyclopedia Britannica How Does Acid Rain Cause Damage When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. Acid rain leaches aluminum from the soil. Dead or dying trees are a common sight in areas effected by acid. How Does Acid Rain Cause Damage.
From ar.inspiredpencil.com
Acid Rain Before And After How Does Acid Rain Cause Damage Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2). Effects of acid rain on plants and trees. Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to. How Does Acid Rain Cause Damage.
From www.youtube.com
Acid Rain How is it formed and what are the effects o the environment How Does Acid Rain Cause Damage How does acid rain affect the environment? That aluminum may be harmful. Some significant consequences are listed below: When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that make the particles acidic can land on statues, buildings, and. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid. How Does Acid Rain Cause Damage.
From hhsvoyager.org
How does acid rain affect the ecosystem? The Voyager How Does Acid Rain Cause Damage Some significant consequences are listed below: Acid rain leaches aluminum from the soil. Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. Typical rain will have a ph of around 5.5 whereas the ph. How Does Acid Rain Cause Damage.
From www.nationalgeographic.com
Acid rain facts and information How Does Acid Rain Cause Damage Dead or dying trees are a common sight in areas effected by acid rain. Acid rain nearly affects everything around us. Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. Acid rain leaches aluminum from the soil. Acid rain can increase the dangers of lakes, rivers, and. How Does Acid Rain Cause Damage.
From www.slideshare.net
Causes of Acid Rain and Its Effects How Does Acid Rain Cause Damage Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2). Some significant consequences are listed below: Effects of acid. How Does Acid Rain Cause Damage.
From ar.inspiredpencil.com
Acid Precipitation Cycle How Does Acid Rain Cause Damage Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to it containing. How Does Acid Rain Cause Damage.
From www.shareyouressays.com
11 Important Effects of Acid Rain on Environment How Does Acid Rain Cause Damage How does acid rain affect the environment? When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that make the particles acidic can land on statues, buildings, and. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no. How Does Acid Rain Cause Damage.
From www.vectorstock.com
Diagram showing acid rain pathway Royalty Free Vector Image How Does Acid Rain Cause Damage Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm. When acid rain falls to the ground and flows into the soil, it absorbs aluminum. How Does Acid Rain Cause Damage.
From www.sciencefacts.net
Acid Rain Definition, Causes, Effects, and Solutions How Does Acid Rain Cause Damage How does acid rain affect the environment? Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2). Effects of acid rain on. How Does Acid Rain Cause Damage.
From www.haikudeck.com
Acid Rain by Megan Forsythe How Does Acid Rain Cause Damage Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2). It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents. How Does Acid Rain Cause Damage.
From radiosargam.com.fj
Acid rain, explained Radio Sargam How Does Acid Rain Cause Damage That aluminum may be harmful. How does acid rain affect the environment? Effects of acid rain on plants and trees. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2). Acid rain can increase the dangers of lakes, rivers, and streams. How Does Acid Rain Cause Damage.
From www.internetgeography.net
What is Acid Rain? Geography How Does Acid Rain Cause Damage Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. That aluminum may be harmful. Effects of acid rain on plants and trees. Some significant consequences are listed below: Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make. How Does Acid Rain Cause Damage.
From www.getbasicidea.com
Acid Rain Environmental Chemistry How Does Acid Rain Cause Damage Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Some significant consequences are listed below: That aluminum may be harmful. Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to it containing dissolved sulphur dioxide or nitrogen oxides. Dead or. How Does Acid Rain Cause Damage.
From thecostaricanews.com
Acid Rain a Serious Threat to the Environment, Product of Pollution ⋆ How Does Acid Rain Cause Damage Effects of acid rain on plants and trees. How does acid rain affect the environment? Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to it containing dissolved sulphur dioxide or. How Does Acid Rain Cause Damage.
From energieadvisor.org
List of 25 Biggest Environmental Issues How Does Acid Rain Cause Damage Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. How does acid rain affect the environment? Acid rain nearly affects everything around us. Acid. How Does Acid Rain Cause Damage.
From earth.org
How Does Acid Rain Affect the Environment How Does Acid Rain Cause Damage That aluminum may be harmful. Acid rain leaches aluminum from the soil. Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen. How Does Acid Rain Cause Damage.
From rain-acid.weebly.com
Causes Acid Rain How Does Acid Rain Cause Damage When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. Dead or dying trees are a common sight in areas effected by acid rain. Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to it containing dissolved sulphur dioxide. How Does Acid Rain Cause Damage.
From www.lisbonlx.com
Definition Of Acid Rain Examples and Forms How Does Acid Rain Cause Damage How does acid rain affect the environment? When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. Some significant consequences are listed below: Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase. How Does Acid Rain Cause Damage.
From www.slideserve.com
PPT Acid Rain PowerPoint Presentation, free download ID2409706 How Does Acid Rain Cause Damage Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm. How does acid rain affect the environment? Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. Acid rain can increase the. How Does Acid Rain Cause Damage.
From znanio.ru
Презентация по английскому языку для учащихся 10 класса "Acid Rain" How Does Acid Rain Cause Damage Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Acid rain leaches aluminum from the soil. How does acid rain affect the environment? That aluminum may be harmful. Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to it containing. How Does Acid Rain Cause Damage.
From pollutantsinthegreatlakes.weebly.com
Acid Rain pollution in the great lakes How Does Acid Rain Cause Damage Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around 4.0 due to it containing dissolved sulphur dioxide or nitrogen oxides. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2).. How Does Acid Rain Cause Damage.
From www.thoughtco.com
Acid Rain Causes, Effects, and Solutions How Does Acid Rain Cause Damage That aluminum may be harmful. Acid rain nearly affects everything around us. Dead or dying trees are a common sight in areas effected by acid rain. When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that. How Does Acid Rain Cause Damage.
From socratic.org
Can acid rain cause damage to the environment? Socratic How Does Acid Rain Cause Damage Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. When acid rain falls to the ground and flows into the soil, it absorbs aluminum from the surrounding. Dead or dying trees. How Does Acid Rain Cause Damage.
From www.teachoo.com
Acid Rain Definition, Causes, Effects Teachoo Concepts How Does Acid Rain Cause Damage Acid rain can increase the dangers of lakes, rivers, and streams more than you might expect. Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen. How Does Acid Rain Cause Damage.
From infographiclist.com
Acid Rain The Consequences [INFOGRAPHIC] Infographic List How Does Acid Rain Cause Damage Acid rain nearly affects everything around us. How does acid rain affect the environment? Effects of acid rain on plants and trees. Some significant consequences are listed below: Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. When acid rain and dry acidic particles fall to earth,. How Does Acid Rain Cause Damage.
From www.worldatlas.com
What Is Acid Rain? WorldAtlas How Does Acid Rain Cause Damage Acid rain leaches aluminum from the soil. When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that make the particles acidic can land on statues, buildings, and. That aluminum may be harmful. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. It robs the. How Does Acid Rain Cause Damage.
From www.breeze-technologies.de
How air pollution causes acid rain Breeze Technologies How Does Acid Rain Cause Damage Acid rain nearly affects everything around us. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a. Acid rain leaches aluminum from the soil. Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. It robs the soil of. How Does Acid Rain Cause Damage.
From www.thoughtco.com
Acid Rain What Is It and How Can You Prevent It? How Does Acid Rain Cause Damage Dead or dying trees are a common sight in areas effected by acid rain. Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm. Typical rain will have a ph of around 5.5 whereas the ph of acid rain is much lower at around. How Does Acid Rain Cause Damage.
From www.thestatesman.com
Acid Rain Causes, consequences and control of the disaster How Does Acid Rain Cause Damage Acid rain, precipitation possessing a ph of about 5.2 or below mainly produced from the emission of sulfur dioxide (so2) and nitrogen oxides (the combination of no and no2). When acid rain and dry acidic particles fall to earth, the nitric and sulfuric acid that make the particles acidic can land on statues, buildings, and. Rotting vegetation and erupting volcanoes. How Does Acid Rain Cause Damage.
From www.teachoo.com
Acid Rain Definition, Causes, Effects Teachoo Concepts How Does Acid Rain Cause Damage Acid deposition can reduce the ph of surface waters, lower biodiversity, and increase the susceptibility of plants to disease and other stressors. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a.. How Does Acid Rain Cause Damage.