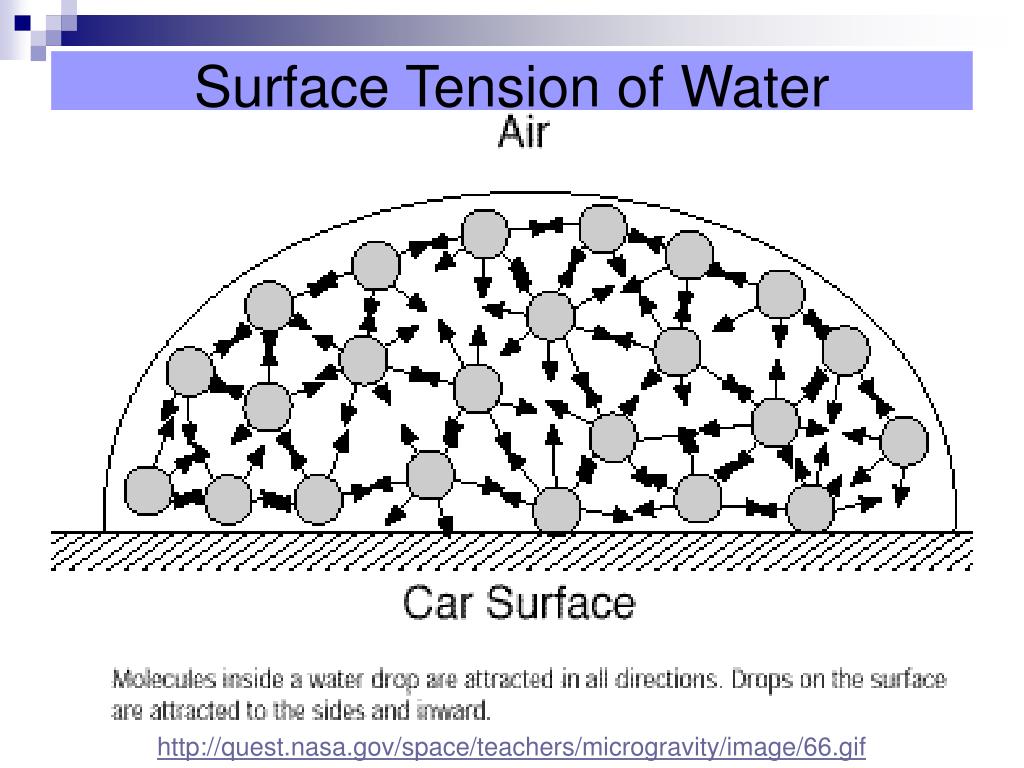
Water Surface Tension Why . Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Check out a few diagrams. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Learn the surface tension of water. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). How does polarity affect it. Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. Does nacl reduce the surface tension of water?
from www.slideserve.com
Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Learn the surface tension of water. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. How does polarity affect it. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Does nacl reduce the surface tension of water? An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. Check out a few diagrams.
PPT Water and Aqueous Systems PowerPoint Presentation ID565121
Water Surface Tension Why Next to mercury, water has the highest surface tension of all commonly occurring liquids. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Does nacl reduce the surface tension of water? Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the. How does polarity affect it. Learn the surface tension of water. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. Check out a few diagrams.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Water Surface Tension Why An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Does nacl reduce the surface tension of. Water Surface Tension Why.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? Water Surface Tension Why Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. How does polarity affect it. Next. Water Surface Tension Why.
From www.biolinscientific.com
Surface tension of water Why is it so high? Water Surface Tension Why Learn the surface tension of water. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them.. Water Surface Tension Why.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Water Surface Tension Why An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. How does polarity affect it. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Learn the surface tension of water. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has. Water Surface Tension Why.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse Water Surface Tension Why Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the. Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively,. Water Surface Tension Why.
From greenearthcleaning.com
Why Is It Better For Clothes? GreenEarth Cleaning Water Surface Tension Why An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the. Learn the surface tension of water.. Water Surface Tension Why.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Water Surface Tension Why Learn the surface tension of water. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the. How does polarity affect it. Next to mercury, water has the highest surface tension of all commonly occurring liquids.. Water Surface Tension Why.
From www.youtube.com
Surface Tension of Water Explained YouTube Water Surface Tension Why Learn the surface tension of water. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Check out a few diagrams. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Surface tension in water might be good at performing tricks, such as being able to float. Water Surface Tension Why.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Water Surface Tension Why Check out a few diagrams. Does nacl reduce the surface tension of water? Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the. Learn the surface tension of water. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Surface. Water Surface Tension Why.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Water Surface Tension Why Learn the surface tension of water. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Does nacl reduce the surface tension of water? Or is the compound distributed evenly and because the. Water Surface Tension Why.
From www.youtube.com
Surface Tension Why are drops spherical? aumsum kids science Water Surface Tension Why Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the. Does nacl reduce the surface tension of water? Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of. Water Surface Tension Why.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Water Surface Tension Why Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. Does nacl reduce the surface tension of water? Water has a surface tension of 0.07275 joule per square metre at 20 °c. Water Surface Tension Why.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with Water Surface Tension Why In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Next to mercury, water has the highest surface tension of all commonly occurring liquids. How does polarity affect it. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its. Water Surface Tension Why.
From www.chegg.com
General Physics for Life Sciences I Water Surface Tension Why Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Learn. Water Surface Tension Why.
From medium.com
Why Freezing Water Floats to the Highest point of a Lake by Haya Khan Water Surface Tension Why Check out a few diagrams. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Does nacl reduce the surface tension of water? How does polarity affect it. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). An. Water Surface Tension Why.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension Water Surface Tension Why Check out a few diagrams. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the. An increase in temperature lowers the. Water Surface Tension Why.
From www.studocu.com
BIOL 1345 Module 2 Water Surface tension is a measure of how hard it Water Surface Tension Why Does nacl reduce the surface tension of water? An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Next. Water Surface Tension Why.
From lessonschoolattollents.z14.web.core.windows.net
Viscosity Vs Surface Tension Water Surface Tension Why In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Check out a few diagrams. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Does nacl reduce the surface tension of water? Surface tension of water can cause things to float which are. Water Surface Tension Why.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Water Surface Tension Why Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. In comparison, organic liquids, such as. Water Surface Tension Why.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Water Surface Tension Why Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the. Next to mercury, water has the highest surface tension of all commonly occurring liquids. How does polarity affect it. An example of such an organism is the water strider, which can run across the surface of water, due to the. Water Surface Tension Why.
From www.youtube.com
The Sci Guys Science at Home SE3 EP4 Water Glass Surface Tension Water Surface Tension Why In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Check out a few diagrams. An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. Or. Water Surface Tension Why.
From learningschoolturbidly.z14.web.core.windows.net
How To Explain Surface Tension Water Surface Tension Why Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. How does polarity affect it. Surface tension of water can cause things to float which are denser than water, allowing organisms to. Water Surface Tension Why.
From www.biolinscientific.com
Surface tension of water Why is it so high? Water Surface Tension Why Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the. Learn the surface tension of water. Check out a few diagrams. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). An increase in temperature lowers. Water Surface Tension Why.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Water Surface Tension Why Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). How does polarity affect it. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the. In comparison, organic liquids,. Water Surface Tension Why.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 Water Surface Tension Why Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties that are vitally important to the. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Next to mercury, water has the highest surface. Water Surface Tension Why.
From ar.inspiredpencil.com
Surface Tension Water Water Surface Tension Why An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Learn the surface tension of water. Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding. Water Surface Tension Why.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Water Surface Tension Why An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). An increase in temperature lowers the net force of attraction. Water Surface Tension Why.
From ar.inspiredpencil.com
Surface Tension Water Water Surface Tension Why An example of such an organism is the water strider, which can run across the surface of water, due to the intermolecular forces of the molecules, and the force of the strider which is. Does nacl reduce the surface tension of water? Surface tension of water can cause things to float which are denser than water, allowing organisms to literally. Water Surface Tension Why.
From www.biolinscientific.com
Why is surface tension important? Water Surface Tension Why Does nacl reduce the surface tension of water? In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the. Water, for example, has a very high surface tension, because oxygen and. Water Surface Tension Why.
From sites.udel.edu
How to Teach Your Kids Science and Engineering K12 Engineering Water Surface Tension Why Check out a few diagrams. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Learn the surface tension of water. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Does nacl reduce the surface tension of water? An example of such an organism is the water strider,. Water Surface Tension Why.
From lexica.art
Lexica Breaking the surface tension of water, photograph Water Surface Tension Why Does nacl reduce the surface tension of water? Learn the surface tension of water. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Water Surface Tension Why.
From mungfali.com
Water Surface Tension Bubble Water Surface Tension Why An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension in water might. Water Surface Tension Why.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Water Surface Tension Why In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Learn the surface tension of water. How does polarity affect it. An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Surface tension in water might be good at performing tricks, such as. Water Surface Tension Why.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension Water Surface Tension Why In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components. Water Surface Tension Why.
From ar.inspiredpencil.com
Surface Tension Water Water Surface Tension Why An increase in temperature lowers the net force of attraction among molecules and hence decreases surface tension. Water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have partial negative and positive charges, respectively, and are thus attracted to all of the other water molecules surrounding them. In comparison, organic liquids, such. Water Surface Tension Why.