What Is Base Used For . Explore common bases such as sodium hydroxide,. Learn what a base is in chemistry and how to classify it based on its properties and reactions. Find out the differences between strong, weak, and alkaline earth metal bases, and. Arrhenius bases, brønsted bases, and lewis bases. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Learn what bases are, how they taste, feel, and react in water, and what they are used for. A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Find out 20 common examples of. In chemistry, there are three definitions in common use of the word base: Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids.
from www.studocu.com
Find out 20 common examples of. Explore common bases such as sodium hydroxide,. A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. In chemistry, there are three definitions in common use of the word base: Find out the differences between strong, weak, and alkaline earth metal bases, and. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Learn what a base is in chemistry and how to classify it based on its properties and reactions. Arrhenius bases, brønsted bases, and lewis bases. Learn what bases are, how they taste, feel, and react in water, and what they are used for.
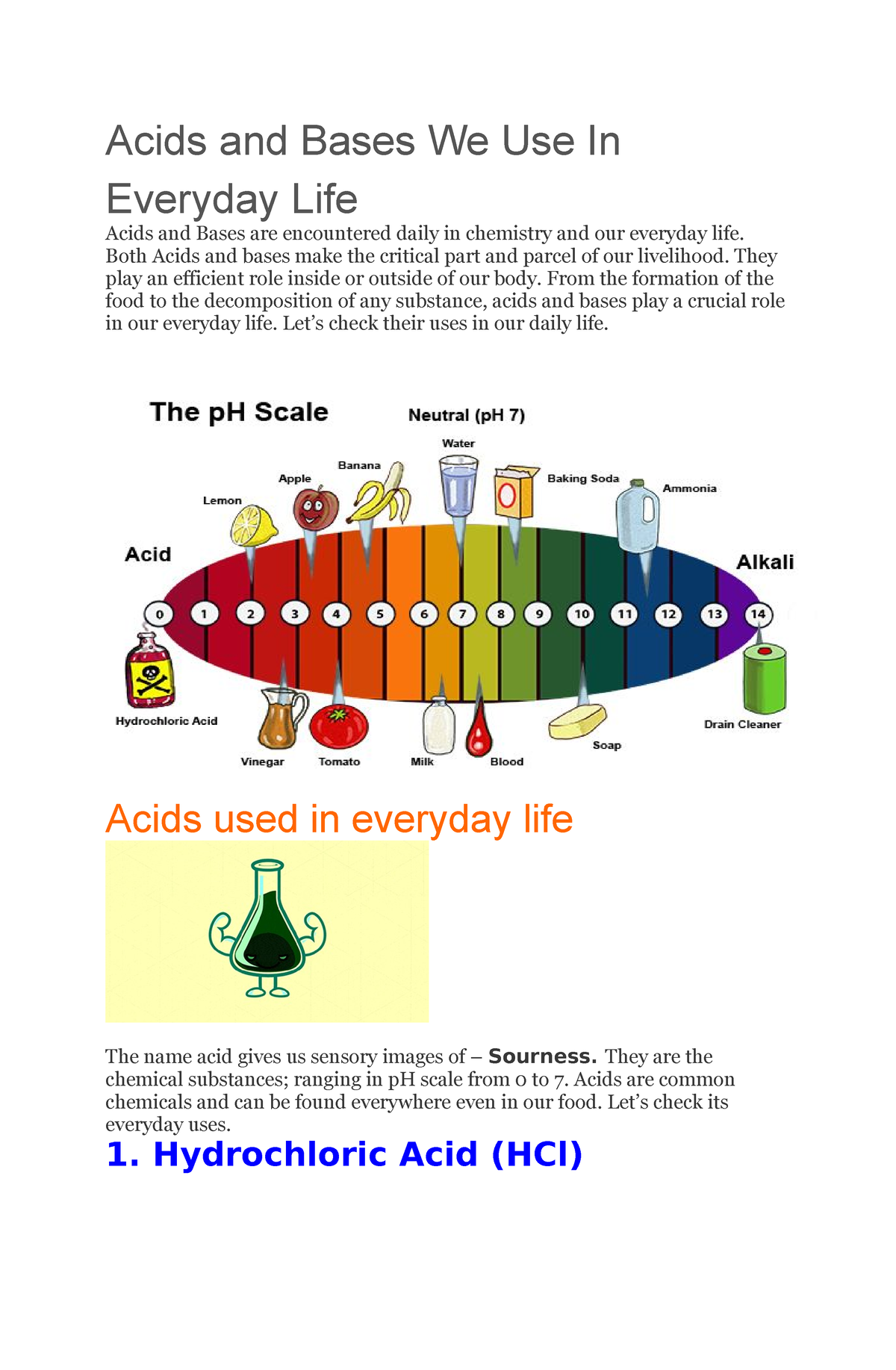
Acids and Bases We Use In Everyday Life Acids and Bases We Use In
What Is Base Used For In chemistry, there are three definitions in common use of the word base: A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Arrhenius bases, brønsted bases, and lewis bases. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Learn what a base is in chemistry and how to classify it based on its properties and reactions. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Find out the differences between strong, weak, and alkaline earth metal bases, and. Learn what bases are, how they taste, feel, and react in water, and what they are used for. In chemistry, there are three definitions in common use of the word base: Find out 20 common examples of. Explore common bases such as sodium hydroxide,.
From gioarkfwz.blob.core.windows.net
What Is An Base Unit at Heather Smalls blog What Is Base Used For Find out 20 common examples of. Arrhenius bases, brønsted bases, and lewis bases. Explore common bases such as sodium hydroxide,. Find out the differences between strong, weak, and alkaline earth metal bases, and. Learn what a base is in chemistry and how to classify it based on its properties and reactions. In chemistry, there are three definitions in common use. What Is Base Used For.
From gioykenos.blob.core.windows.net
What Is A Base Decimal System at Mark Yerby blog What Is Base Used For In chemistry, there are three definitions in common use of the word base: Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Explore common bases such as sodium hydroxide,. Arrhenius bases, brønsted bases, and lewis bases. Learn what bases are, how they taste, feel, and react in water, and. What Is Base Used For.
From praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs What Is Base Used For Explore common bases such as sodium hydroxide,. Arrhenius bases, brønsted bases, and lewis bases. Find out 20 common examples of. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Find out the differences between strong, weak, and alkaline earth metal bases, and. Learn what bases are, how they taste,. What Is Base Used For.
From www.vrogue.co
What Is A Common Use Of Bases vrogue.co What Is Base Used For A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Find out the differences between strong, weak, and alkaline earth metal bases, and. Explore common bases such as sodium hydroxide,. Learn what bases are, how they taste, feel, and react in water, and what. What Is Base Used For.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo What Is Base Used For Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Explore common bases such as sodium hydroxide,. Find out the differences between strong, weak, and alkaline earth metal bases, and. Arrhenius bases, brønsted bases, and lewis bases. A base is a substance that reacts with acids to form salts and. What Is Base Used For.
From giosaarwa.blob.core.windows.net
What Is Makeup Base And Primer at Virginia Kerr blog What Is Base Used For Arrhenius bases, brønsted bases, and lewis bases. In chemistry, there are three definitions in common use of the word base: A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Find out the differences between strong, weak, and alkaline earth metal bases, and. A. What Is Base Used For.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation, free download What Is Base Used For Learn what bases are, how they taste, feel, and react in water, and what they are used for. A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Find out the differences between strong, weak, and alkaline earth metal bases, and. Find out 20. What Is Base Used For.
From www.slideshare.net
Acid & bases What Is Base Used For In chemistry, there are three definitions in common use of the word base: Find out the differences between strong, weak, and alkaline earth metal bases, and. Learn what a base is in chemistry and how to classify it based on its properties and reactions. A base is a substance that reacts with acids to form a salt and which releases. What Is Base Used For.
From giosaarwa.blob.core.windows.net
What Is Makeup Base And Primer at Virginia Kerr blog What Is Base Used For In chemistry, there are three definitions in common use of the word base: Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Arrhenius bases, brønsted bases, and lewis bases. Find out the differences between strong, weak, and alkaline earth metal bases, and. Find out 20 common examples of. Learn. What Is Base Used For.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID9013474 What Is Base Used For A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Learn what bases are, how they taste, feel, and react in water, and what they are used for. Learn what a base is in chemistry and how to classify it based on its properties and reactions. Arrhenius bases, brønsted bases,. What Is Base Used For.
From civilcivstudio.blogspot.com
Elastomeric Bearing Pad CIVILCIV What Is Base Used For Explore common bases such as sodium hydroxide,. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. A base is a substance that reacts with acids to form a salt. What Is Base Used For.
From definitionklw.blogspot.com
Definition Of Base In Science DEFINITION KLW What Is Base Used For Learn what bases are, how they taste, feel, and react in water, and what they are used for. In chemistry, there are three definitions in common use of the word base: Explore common bases such as sodium hydroxide,. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Arrhenius bases,. What Is Base Used For.
From www.yubrain.com
What is an acid base indicator? What Is Base Used For Find out 20 common examples of. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Explore common bases such as sodium hydroxide,. Find out the differences between strong, weak, and alkaline earth metal bases, and. Arrhenius bases, brønsted bases, and lewis bases. A base is a substance that reacts. What Is Base Used For.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6523668 What Is Base Used For A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. In chemistry, there are three definitions in common use of the word base: A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Find. What Is Base Used For.
From www.vrogue.co
What Is A Common Use Of Bases vrogue.co What Is Base Used For A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Learn what a base is in chemistry and how to classify it based on its properties and reactions. Find out 20 common examples of. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper. What Is Base Used For.
From read.cholonautas.edu.pe
What Is A Base Class Printable Templates Free What Is Base Used For Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Explore common bases such as sodium hydroxide,. Learn what bases are, how they taste, feel, and react in water, and what they are used for. Arrhenius bases, brønsted bases, and lewis bases. Learn what a base is in chemistry and. What Is Base Used For.
From www.studocu.com
Acids and Bases We Use In Everyday Life Acids and Bases We Use In What Is Base Used For In chemistry, there are three definitions in common use of the word base: Learn what a base is in chemistry and how to classify it based on its properties and reactions. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Learn about the characteristics, uses, and reactions of bases,. What Is Base Used For.
From www.teachoo.com
Uses of Base 5+ Examples Chemistry Teachoo Teachoo Questions What Is Base Used For Arrhenius bases, brønsted bases, and lewis bases. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Learn what bases are, how they taste, feel, and react in water, and what they are used for. Find out 20 common examples of. Find out the differences between strong, weak, and alkaline. What Is Base Used For.
From sciencenotes.org
SI Base Units What Is Base Used For Explore common bases such as sodium hydroxide,. Arrhenius bases, brønsted bases, and lewis bases. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. In chemistry, there are three definitions in common use of the word base: Find out 20 common examples of. Learn what a base is in chemistry. What Is Base Used For.
From gionwwgys.blob.core.windows.net
What Is The Meaning Base Map at Kim Wilson blog What Is Base Used For Explore common bases such as sodium hydroxide,. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Arrhenius bases, brønsted bases, and lewis bases. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. In chemistry, there are three definitions. What Is Base Used For.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5349215 What Is Base Used For A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Learn what a base is in chemistry and how to classify it based on. What Is Base Used For.
From examples.yourdictionary.com
Difference Between Acids and Bases Key Properties What Is Base Used For Arrhenius bases, brønsted bases, and lewis bases. Learn what a base is in chemistry and how to classify it based on its properties and reactions. Find out the differences between strong, weak, and alkaline earth metal bases, and. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Find out. What Is Base Used For.
From gioarkfwz.blob.core.windows.net
What Is An Base Unit at Heather Smalls blog What Is Base Used For A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Find out 20 common examples of. Explore common bases such as sodium hydroxide,. Learn what bases are, how they taste, feel, and react in water, and what they are used for. Learn about the. What Is Base Used For.
From www.slideserve.com
PPT CHEMISTRY REVIEW PowerPoint Presentation, free download ID4920836 What Is Base Used For Find out 20 common examples of. In chemistry, there are three definitions in common use of the word base: A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Find. What Is Base Used For.
From gioarkfwz.blob.core.windows.net
What Is An Base Unit at Heather Smalls blog What Is Base Used For In chemistry, there are three definitions in common use of the word base: Explore common bases such as sodium hydroxide,. Find out 20 common examples of. Find out the differences between strong, weak, and alkaline earth metal bases, and. Learn what bases are, how they taste, feel, and react in water, and what they are used for. Learn about the. What Is Base Used For.
From giosaarwa.blob.core.windows.net
What Is Makeup Base And Primer at Virginia Kerr blog What Is Base Used For Find out 20 common examples of. Learn what a base is in chemistry and how to classify it based on its properties and reactions. Explore common bases such as sodium hydroxide,. Learn what bases are, how they taste, feel, and react in water, and what they are used for. A base is a substance that reacts with acids to form. What Is Base Used For.
From gioykenos.blob.core.windows.net
What Is A Base Decimal System at Mark Yerby blog What Is Base Used For Explore common bases such as sodium hydroxide,. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Learn what bases are, how they taste, feel, and react in water, and what they are used for. A base is a substance that reacts with acids to form a salt and which. What Is Base Used For.
From www.worksheetsplanet.com
What is a Base Definition of Base What Is Base Used For Arrhenius bases, brønsted bases, and lewis bases. Find out the differences between strong, weak, and alkaline earth metal bases, and. Learn what bases are, how they taste, feel, and react in water, and what they are used for. Find out 20 common examples of. Learn what a base is in chemistry and how to classify it based on its properties. What Is Base Used For.
From gionwwgys.blob.core.windows.net
What Is The Meaning Base Map at Kim Wilson blog What Is Base Used For Find out the differences between strong, weak, and alkaline earth metal bases, and. Find out 20 common examples of. Learn what bases are, how they taste, feel, and react in water, and what they are used for. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Explore common bases. What Is Base Used For.
From www.slideserve.com
PPT Chapter 23 Acids, Bases, and Salts PowerPoint Presentation, free What Is Base Used For Learn what a base is in chemistry and how to classify it based on its properties and reactions. Find out the differences between strong, weak, and alkaline earth metal bases, and. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. In chemistry, there are three definitions in common use. What Is Base Used For.
From ar.inspiredpencil.com
Example Of Chemical Base What Is Base Used For A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Explore common bases such as sodium hydroxide,. Learn what bases are, how they taste,. What Is Base Used For.
From sciencenotes.org
What Is a Base in Chemistry? Definition and Examples What Is Base Used For In chemistry, there are three definitions in common use of the word base: Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Learn. What Is Base Used For.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo What Is Base Used For Explore common bases such as sodium hydroxide,. Learn what bases are, how they taste, feel, and react in water, and what they are used for. A base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. In chemistry, there are three definitions in common use. What Is Base Used For.
From giosaarwa.blob.core.windows.net
What Is Makeup Base And Primer at Virginia Kerr blog What Is Base Used For In chemistry, there are three definitions in common use of the word base: Learn what a base is in chemistry and how to classify it based on its properties and reactions. Learn about the characteristics, uses, and reactions of bases, which are substances that turn litmus paper blue and neutralize acids. Explore common bases such as sodium hydroxide,. A base. What Is Base Used For.
From www.slideserve.com
PPT Common Bases PowerPoint Presentation, free download ID2782494 What Is Base Used For Find out 20 common examples of. Explore common bases such as sodium hydroxide,. Find out the differences between strong, weak, and alkaline earth metal bases, and. A base is a substance that reacts with acids to form salts and has other characteristic properties in water solutions. Learn about the characteristics, uses, and reactions of bases, which are substances that turn. What Is Base Used For.