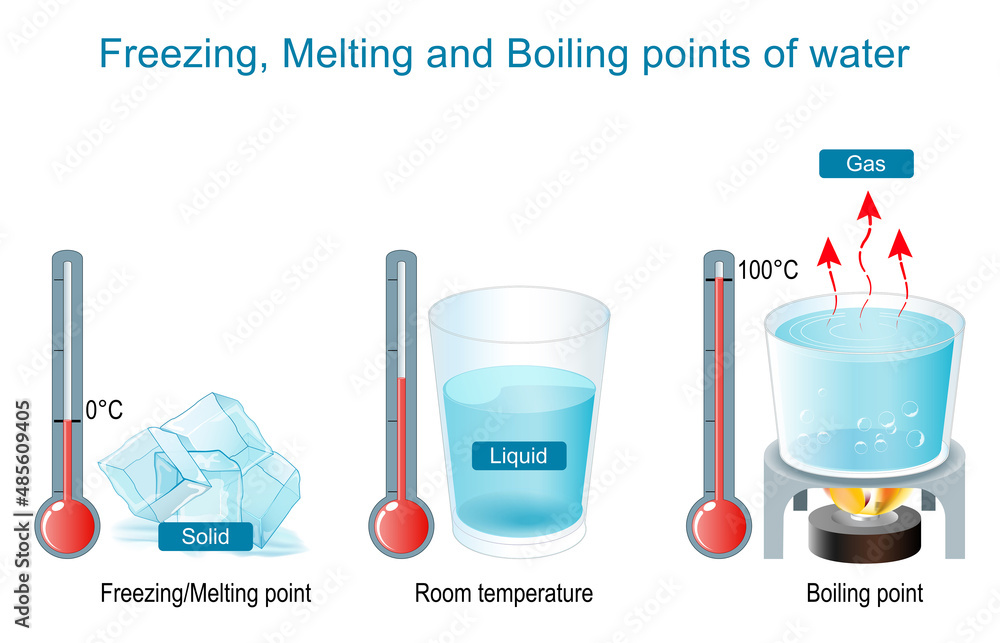
What Is The Boiling Point Of Liquid . The reason boiling point changes with elevation is because. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. What is a boiling point? The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The boiling point is the temperature at which boiling occurs for a specific liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point which is usually quoted in chemical literature. For example, for water, the boiling point is 100ºc at. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point.
from stock.adobe.com
The reason boiling point changes with elevation is because. For example, for water, the boiling point is 100ºc at. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. What is a boiling point? This is the boiling point which is usually quoted in chemical literature. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The boiling point is the temperature at which boiling occurs for a specific liquid.
Boiling and Evaporation, Freezing and Melting Points of Water. Stock
What Is The Boiling Point Of Liquid Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. This is the boiling point which is usually quoted in chemical literature. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. The boiling point is the temperature at which boiling occurs for a specific liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. The reason boiling point changes with elevation is because. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. What is a boiling point? For example, for water, the boiling point is 100ºc at. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID What Is The Boiling Point Of Liquid What is a boiling point? The reason boiling point changes with elevation is because. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The boiling point of a substance is the temperature at which the vapor. What Is The Boiling Point Of Liquid.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is The Boiling Point Of Liquid Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The reason boiling point changes with elevation is because. Equal to one atmosphere, the boiling. What Is The Boiling Point Of Liquid.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Is The Boiling Point Of Liquid The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. What is a boiling point? This is the boiling point which is usually quoted in chemical literature. For example, for water, the boiling point is 100ºc at. The reason boiling point changes with elevation is because. The boiling point. What Is The Boiling Point Of Liquid.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is The Boiling Point Of Liquid The boiling point is the temperature at which boiling occurs for a specific liquid. For example, for water, the boiling point is 100ºc at. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling point of a substance is the. What Is The Boiling Point Of Liquid.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is The Boiling Point Of Liquid The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. The reason boiling point changes with. What Is The Boiling Point Of Liquid.
From marshallkruwmathis.blogspot.com
What is the Boiling Point of Water MarshallkruwMathis What Is The Boiling Point Of Liquid What is a boiling point? For example, for water, the boiling point is 100ºc at. The reason boiling point changes with elevation is because. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils. What Is The Boiling Point Of Liquid.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Boiling Point Of Liquid The boiling point is the temperature at which boiling occurs for a specific liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. This is the boiling point which is usually quoted in chemical literature. Equal to one atmosphere, the boiling point of a liquid is called. What Is The Boiling Point Of Liquid.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is The Boiling Point Of Liquid For example, for water, the boiling point is 100ºc at. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which. What Is The Boiling Point Of Liquid.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Is The Boiling Point Of Liquid This is the boiling point which is usually quoted in chemical literature. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The reason boiling point changes with elevation is because. The boiling point of a substance is the temperature at which the vapor pressure of the liquid. What Is The Boiling Point Of Liquid.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is The Boiling Point Of Liquid Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. The reason boiling point changes with elevation is. What Is The Boiling Point Of Liquid.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Is The Boiling Point Of Liquid Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. This is the boiling point which. What Is The Boiling Point Of Liquid.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Is The Boiling Point Of Liquid The boiling point is the temperature at which boiling occurs for a specific liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. This. What Is The Boiling Point Of Liquid.
From www.youtube.com
CHEMISTRY 201 Calculating Boiling Point Using Clausius Clapeyron What Is The Boiling Point Of Liquid What is a boiling point? This is the boiling point which is usually quoted in chemical literature. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The boiling point of a substance is the temperature at. What Is The Boiling Point Of Liquid.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and What Is The Boiling Point Of Liquid Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. For example, for water, the boiling point is 100ºc at. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. What is a boiling point? This is the boiling point which is usually quoted in chemical literature.. What Is The Boiling Point Of Liquid.
From mungfali.com
Water Boiling Point Pressure Chart What Is The Boiling Point Of Liquid Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The boiling point is the temperature at which boiling occurs for a specific liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as. What Is The Boiling Point Of Liquid.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Is The Boiling Point Of Liquid The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. What is a boiling point? The reason boiling point changes with elevation is because. The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of a substance is the temperature at which the vapor pressure of the. What Is The Boiling Point Of Liquid.
From blog.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks What Is The Boiling Point Of Liquid The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. This is the boiling point which is usually quoted in chemical literature. The boiling point is the temperature at which boiling occurs. What Is The Boiling Point Of Liquid.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and What Is The Boiling Point Of Liquid The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred. What Is The Boiling Point Of Liquid.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes What Is The Boiling Point Of Liquid The reason boiling point changes with elevation is because. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Equal to one atmosphere, the boiling point of a liquid is called the normal. What Is The Boiling Point Of Liquid.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 What Is The Boiling Point Of Liquid The reason boiling point changes with elevation is because. This is the boiling point which is usually quoted in chemical literature. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The boiling point is the temperature. What Is The Boiling Point Of Liquid.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Is The Boiling Point Of Liquid Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. What is a boiling point? The boiling point of. What Is The Boiling Point Of Liquid.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is The Boiling Point Of Liquid The reason boiling point changes with elevation is because. For example, for water, the boiling point is 100ºc at. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. This is the boiling. What Is The Boiling Point Of Liquid.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Is The Boiling Point Of Liquid This is the boiling point which is usually quoted in chemical literature. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of water is. What Is The Boiling Point Of Liquid.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting What Is The Boiling Point Of Liquid The reason boiling point changes with elevation is because. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. This is the boiling point which is. What Is The Boiling Point Of Liquid.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is The Boiling Point Of Liquid The reason boiling point changes with elevation is because. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. What is a boiling point? If this pressure is the standard pressure of 1 atm (101.3 kpa), then. What Is The Boiling Point Of Liquid.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Is The Boiling Point Of Liquid For example, for water, the boiling point is 100ºc at. What is a boiling point? Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. The boiling point of water is the temperature where the liquid’s vapor. What Is The Boiling Point Of Liquid.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is The Boiling Point Of Liquid Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of water is the temperature where the liquid’s vapor pressure. What Is The Boiling Point Of Liquid.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Boiling Point Of Liquid This is the boiling point which is usually quoted in chemical literature. The boiling point is the temperature at which boiling occurs for a specific liquid. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by. What Is The Boiling Point Of Liquid.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock What Is The Boiling Point Of Liquid The boiling point is the temperature at which boiling occurs for a specific liquid. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. If this pressure is the standard pressure of. What Is The Boiling Point Of Liquid.
From www.gettyimages.ie
Freezing And Boiling Points In Celsius And Fahrenheit HighRes Vector What Is The Boiling Point Of Liquid This is the boiling point which is usually quoted in chemical literature. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Greater than one atmosphere, the boiling point of the liquid is greater than its normal boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is. What Is The Boiling Point Of Liquid.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of What Is The Boiling Point Of Liquid What is a boiling point? The boiling point is the temperature at which boiling occurs for a specific liquid. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The reason boiling. What Is The Boiling Point Of Liquid.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) What Is The Boiling Point Of Liquid The reason boiling point changes with elevation is because. This is the boiling point which is usually quoted in chemical literature. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. If this. What Is The Boiling Point Of Liquid.
From www.animalia-life.club
Boiling Point Of Water For Kids What Is The Boiling Point Of Liquid The reason boiling point changes with elevation is because. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The boiling point is the temperature at which boiling occurs for a specific liquid. For example, for water, the boiling point is 100ºc. What Is The Boiling Point Of Liquid.
From howchimp.com
What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit What Is The Boiling Point Of Liquid Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The boiling point is the temperature at which boiling occurs for a specific liquid. The reason boiling point changes with elevation is because. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling. What Is The Boiling Point Of Liquid.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Is The Boiling Point Of Liquid The reason boiling point changes with elevation is because. Equal to one atmosphere, the boiling point of a liquid is called the normal boiling point. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. What Is The Boiling Point Of Liquid.