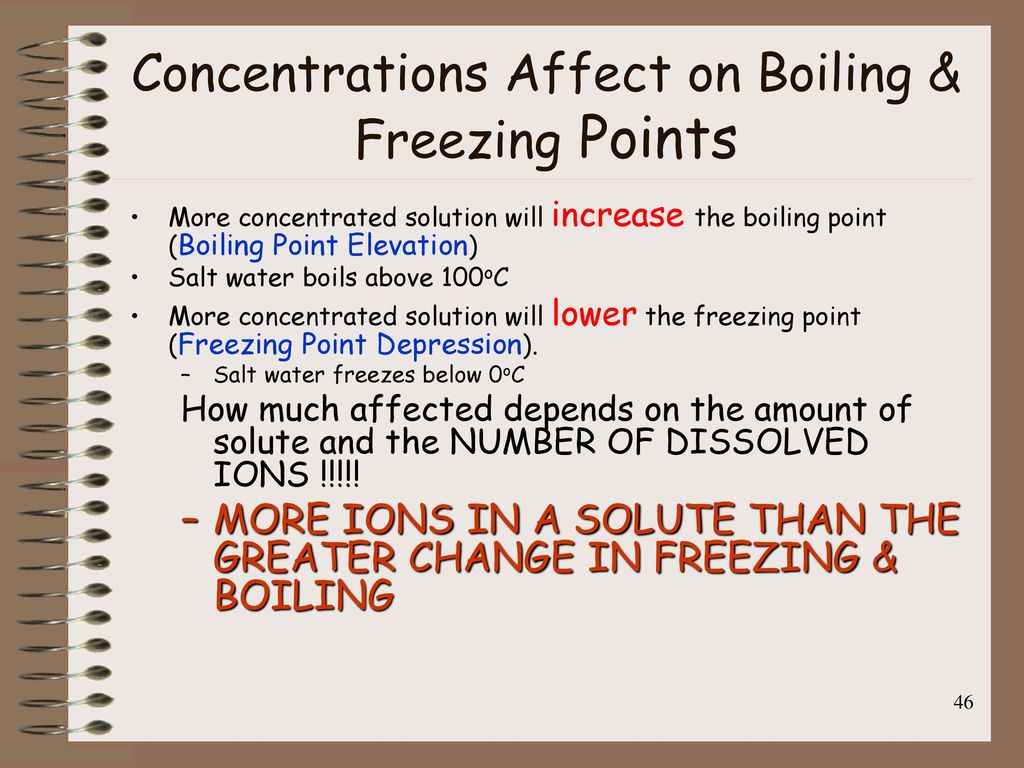
How Does Concentration Affect Freezing Point . The presence of a solute lowers the freezing point of any solvent; The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of. What is the concentration of ethylene glycol in a. The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s).
from slideplayer.com
The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). What is the concentration of ethylene glycol in a. Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The presence of a solute lowers the freezing point of any solvent; The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of.
Water and Aqueous Systems ppt download
How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of. The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). The presence of a solute lowers the freezing point of any solvent; The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. What is the concentration of ethylene glycol in a.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Elevation Chemistry Notes How Does Concentration Affect Freezing Point The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for. How Does Concentration Affect Freezing Point.
From www.thoughtco.com
What Is the Freezing Point of Water? How Does Concentration Affect Freezing Point The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure. How Does Concentration Affect Freezing Point.
From slideplayer.com
Water and Aqueous Systems ppt download How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. The freezing point depression δt f is a colligative property of the solution, and. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Activity 2. Freezing Point of Liquids PowerPoint Presentation, free download ID2158318 How Does Concentration Affect Freezing Point The presence of a solute lowers the freezing point of any solvent; Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of. Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. The freezing. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID6856680 How Does Concentration Affect Freezing Point The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The freezing point of the solvent. How Does Concentration Affect Freezing Point.
From www.researchgate.net
Freezing and thawing points of samples saturated with different NaCl... Download Scientific How Does Concentration Affect Freezing Point The presence of a solute lowers the freezing point of any solvent; Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of. Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. Changes in. How Does Concentration Affect Freezing Point.
From slideplayer.com
Aim How does the addition of a solute affect the colligative properties of the solvent? Do Now How Does Concentration Affect Freezing Point The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. What is the concentration of ethylene glycol in a. The presence of a solute lowers the freezing point of any solvent; Freezing point depression is the lowering of the equilibrium freezing or melting temperature by. How Does Concentration Affect Freezing Point.
From www.researchgate.net
A schematic illustration of maximum freeze concentration in sucrose... Download Scientific Diagram How Does Concentration Affect Freezing Point Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. What is the concentration of ethylene glycol in a. The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation ID6060261 How Does Concentration Affect Freezing Point The presence of a solute lowers the freezing point of any solvent; What is the concentration of ethylene glycol in a. The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Physical Properties of Solutions Review and Colligative Properties PowerPoint Presentation How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly. How Does Concentration Affect Freezing Point.
From chemistnotes.com
Depression of Freezing Point Equation, Definition, and Applications Chemistry Notes How Does Concentration Affect Freezing Point Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. What is the concentration of ethylene glycol in a. The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the. How Does Concentration Affect Freezing Point.
From slideplayer.com
Lesson 6.1 Solutions and Concentration ppt download How Does Concentration Affect Freezing Point What is the concentration of ethylene glycol in a. The presence of a solute lowers the freezing point of any solvent; Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of. Changes. How Does Concentration Affect Freezing Point.
From general.chemistrysteps.com
Freezing Point Depression Chemistry Steps How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). The presence of a solute lowers. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Chapter 16 “ Solutions ” PowerPoint Presentation, free download ID1910799 How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of. The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional. How Does Concentration Affect Freezing Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Water! How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. What is the concentration of ethylene glycol in a. The presence of a solute lowers the freezing point of any solvent; The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). Changes in the freezing point and boiling point of a solution. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Determining the Molecular Mass by Freezing Point Depression PowerPoint Presentation ID How Does Concentration Affect Freezing Point The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. What is the concentration of ethylene glycol in a. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Osmotic pressure and changes in freezing point,. How Does Concentration Affect Freezing Point.
From www.numerade.com
SOLVED As the concentration of a solute in a solution increases, the freezing point of the How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The presence of a solute lowers the freezing point of any solvent; Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. Osmotic pressure. How Does Concentration Affect Freezing Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Water! MTS Blog How Does Concentration Affect Freezing Point What is the concentration of ethylene glycol in a. The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Unlike the boiling point, the chemical potential. How Does Concentration Affect Freezing Point.
From www.jove.com
11369.jpg How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. What is the concentration of ethylene glycol in a. The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. Unlike the boiling point, the chemical potential. How Does Concentration Affect Freezing Point.
From www.researchgate.net
The relationship between the concentration of ethanol and its freezing... Download Scientific How Does Concentration Affect Freezing Point Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of. Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. The freezing point of the solvent in a solution changes as the concentration of. How Does Concentration Affect Freezing Point.
From sciencenotes.org
What Is the Freezing Point of Water? Fahrenheit, Celsius, and Kelvin How Does Concentration Affect Freezing Point The presence of a solute lowers the freezing point of any solvent; Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional. How Does Concentration Affect Freezing Point.
From sciencenotes.org
Freezing Point Depression Formula and Definition How Does Concentration Affect Freezing Point Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure solid solvent. The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID4365473 How Does Concentration Affect Freezing Point The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. Freezing point depression is the lowering of the equilibrium. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Effects of solute on solvent PowerPoint Presentation, free download ID6556425 How Does Concentration Affect Freezing Point The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). What is the concentration of ethylene glycol in a. Changes in the freezing point and boiling. How Does Concentration Affect Freezing Point.
From www.researchgate.net
Freezing and melting points of mortar specimens saturated with... Download Scientific Diagram How Does Concentration Affect Freezing Point The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of. The freezing point of the solvent in a solution changes as the concentration of the solute. How Does Concentration Affect Freezing Point.
From facts.net
12 Extraordinary Facts About Freezing Point Depression How Does Concentration Affect Freezing Point The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Changes in the freezing point and. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation ID6060261 How Does Concentration Affect Freezing Point The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature for it to reach the chemical potential of the pure. How Does Concentration Affect Freezing Point.
From www.mdpi.com
Water Free FullText Investigation into Freezing Point Depression in Soil Caused by NaCl How Does Concentration Affect Freezing Point The presence of a solute lowers the freezing point of any solvent; Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. The main component. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation ID6060261 How Does Concentration Affect Freezing Point The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Osmotic pressure and changes in freezing. How Does Concentration Affect Freezing Point.
From chem.libretexts.org
13.8 FreezingPoint Depression and BoilingPoint Elevation of Nonelectrolyte Solutions How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The presence of a solute lowers the freezing point of any solvent; The freezing point depression δt f is a colligative property of the solution, and for dilute solutions is found to be proportional to the molal. The main component in. How Does Concentration Affect Freezing Point.
From chem.libretexts.org
13.8 FreezingPoint Depression and BoilingPoint Elevation of Nonelectrolyte Solutions How Does Concentration Affect Freezing Point Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the concentration of. Unlike the boiling point, the chemical potential of the impure solvent requires a colder temperature. How Does Concentration Affect Freezing Point.
From general.chemistrysteps.com
Freezing Point Depression Chemistry Steps How Does Concentration Affect Freezing Point The presence of a solute lowers the freezing point of any solvent; The freezing point of the solvent in a solution changes as the concentration of the solute in the solution changes (but it does not depend on the identity of either the solvent or the solute(s). What is the concentration of ethylene glycol in a. Changes in the freezing. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation ID6060261 How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The presence of a solute lowers the freezing point of any solvent; Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. What is the. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT How is freezing point affected by different ratios of solute to solvent? PowerPoint How Does Concentration Affect Freezing Point The main component in antifreeze is ethylene glycol, \(\ce{c_2h_4(oh)_2}\). Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. The presence of a solute lowers the freezing point of any solvent; What is the concentration of ethylene glycol in a. Changes in the freezing point and boiling point of a solution. How Does Concentration Affect Freezing Point.
From www.slideserve.com
PPT Determining the Molecular Mass by Freezing Point Depression PowerPoint Presentation ID How Does Concentration Affect Freezing Point Freezing point depression is the lowering of the equilibrium freezing or melting temperature by solutes in the liquid phase. Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. The freezing point of the solvent in a solution changes as the concentration of the. How Does Concentration Affect Freezing Point.