What Is The Boiling Point Glycerine . This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Learn about the structure, properties, and uses of glycerin here. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Thus ethylene glycol does not boil away when it is used as an antifreeze. Individual data points quantity value units. Quantity value units method reference comment; Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: The freezing points are reduced until glycerine concentration is. Experimental densities at pressures of up to 25 mpa, and. This results in a high boiling point—198°c; The boiling point of glycerin is 290°c.
from chemistnotes.com
Thus ethylene glycol does not boil away when it is used as an antifreeze. Individual data points quantity value units. Learn about the structure, properties, and uses of glycerin here. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: This results in a high boiling point—198°c; Quantity value units method reference comment; The freezing points are reduced until glycerine concentration is. Experimental densities at pressures of up to 25 mpa, and. This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine.
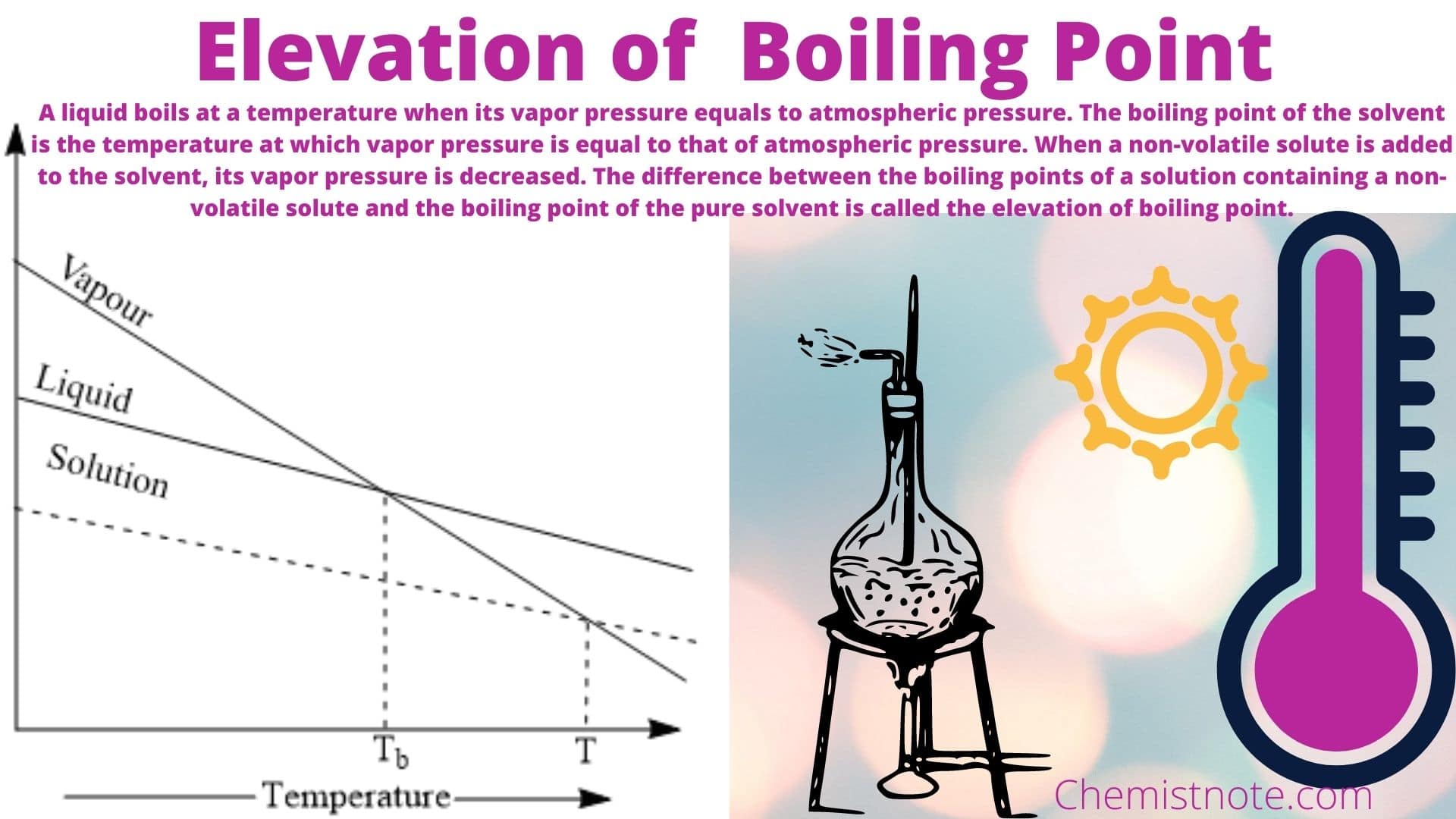
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Elevation Chemistry Notes
What Is The Boiling Point Glycerine The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: The boiling point of glycerin is 290°c. This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Thus ethylene glycol does not boil away when it is used as an antifreeze. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Learn about the structure, properties, and uses of glycerin here. Experimental densities at pressures of up to 25 mpa, and. The freezing points are reduced until glycerine concentration is. Quantity value units method reference comment; Individual data points quantity value units. This results in a high boiling point—198°c;
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube What Is The Boiling Point Glycerine Experimental densities at pressures of up to 25 mpa, and. The boiling point of glycerin is 290°c. Quantity value units method reference comment; This results in a high boiling point—198°c; The freezing points are reduced until glycerine concentration is. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: Learn. What Is The Boiling Point Glycerine.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting boiling point of liquids What Is The Boiling Point Glycerine Learn about the structure, properties, and uses of glycerin here. This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: The freezing points are reduced until glycerine concentration is. Individual data points quantity value. What Is The Boiling Point Glycerine.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point Formula Boiling Points YouTube What Is The Boiling Point Glycerine The freezing points are reduced until glycerine concentration is. Experimental densities at pressures of up to 25 mpa, and. This results in a high boiling point—198°c; Thus ethylene glycol does not boil away when it is used as an antifreeze. Quantity value units method reference comment; Individual data points quantity value units. Improving a variation of the dsc technique for. What Is The Boiling Point Glycerine.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Boiling Point Glycerine This results in a high boiling point—198°c; Thus ethylene glycol does not boil away when it is used as an antifreeze. Experimental densities at pressures of up to 25 mpa, and. The freezing points are reduced until glycerine concentration is. Quantity value units method reference comment; Individual data points quantity value units. This high boiling point is a result of. What Is The Boiling Point Glycerine.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Experiments What Is The Boiling Point Glycerine Experimental densities at pressures of up to 25 mpa, and. Learn about the structure, properties, and uses of glycerin here. Quantity value units method reference comment; Individual data points quantity value units. This results in a high boiling point—198°c; Thus ethylene glycol does not boil away when it is used as an antifreeze. The freezing points are reduced until glycerine. What Is The Boiling Point Glycerine.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Elevation Chemistry Notes What Is The Boiling Point Glycerine Experimental densities at pressures of up to 25 mpa, and. The freezing points are reduced until glycerine concentration is. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Learn about the structure, properties, and uses of glycerin here. Thus ethylene glycol does not boil away when it is used as. What Is The Boiling Point Glycerine.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is The Boiling Point Glycerine The freezing points are reduced until glycerine concentration is. Quantity value units method reference comment; This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Experimental densities at pressures of up to 25 mpa, and. Learn about the structure, properties, and uses of glycerin here. The boiling point of glycerin is 290°c. Improving a. What Is The Boiling Point Glycerine.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Is The Boiling Point Glycerine Individual data points quantity value units. Thus ethylene glycol does not boil away when it is used as an antifreeze. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: Quantity value units method reference comment; Experimental densities at pressures of up to 25 mpa, and. This results in a. What Is The Boiling Point Glycerine.
From community.ptc.com
Boiling Point Elevation PTC Community What Is The Boiling Point Glycerine Quantity value units method reference comment; Experimental densities at pressures of up to 25 mpa, and. Individual data points quantity value units. This results in a high boiling point—198°c; The freezing points are reduced until glycerine concentration is. Learn about the structure, properties, and uses of glycerin here. Improving a variation of the dsc technique for measuring the boiling points. What Is The Boiling Point Glycerine.
From europepmc.org
Boiling points of the propylene glycol + glycerol system at 1 atmosphere pressure 188.6292 °C What Is The Boiling Point Glycerine This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. The boiling point of glycerin is 290°c. Thus ethylene glycol does not boil away when it is used as an antifreeze. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Learn about the structure,. What Is The Boiling Point Glycerine.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free download ID2755993 What Is The Boiling Point Glycerine This results in a high boiling point—198°c; This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Thus ethylene glycol does not boil away when it is used as an antifreeze. Individual data points quantity value units. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts. What Is The Boiling Point Glycerine.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is The Boiling Point Glycerine Thus ethylene glycol does not boil away when it is used as an antifreeze. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Learn about the structure, properties, and uses of glycerin here.. What Is The Boiling Point Glycerine.
From www.researchgate.net
Physicochemical properties of glycerol. Download Table What Is The Boiling Point Glycerine Individual data points quantity value units. This results in a high boiling point—198°c; The boiling point of glycerin is 290°c. Experimental densities at pressures of up to 25 mpa, and. Learn about the structure, properties, and uses of glycerin here. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol:. What Is The Boiling Point Glycerine.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is The Boiling Point Glycerine This results in a high boiling point—198°c; Quantity value units method reference comment; The boiling point of glycerin is 290°c. Learn about the structure, properties, and uses of glycerin here. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Experimental densities at pressures of up to 25 mpa, and. Improving. What Is The Boiling Point Glycerine.
From www.peoi.org
Chapter 10 Section C Properties of Liquids What Is The Boiling Point Glycerine Learn about the structure, properties, and uses of glycerin here. Individual data points quantity value units. Experimental densities at pressures of up to 25 mpa, and. This results in a high boiling point—198°c; This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Quantity value units method reference comment; Improving a variation of the. What Is The Boiling Point Glycerine.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry What Is The Boiling Point Glycerine The boiling point of glycerin is 290°c. This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Experimental densities at pressures of up to 25 mpa, and. The freezing points are reduced until glycerine concentration is. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures. What Is The Boiling Point Glycerine.
From www.chemistrysteps.com
Boiling Point and Melting Point Practice Problems Chemistry Steps What Is The Boiling Point Glycerine The freezing points are reduced until glycerine concentration is. Learn about the structure, properties, and uses of glycerin here. Thus ethylene glycol does not boil away when it is used as an antifreeze. Quantity value units method reference comment; This results in a high boiling point—198°c; The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced. What Is The Boiling Point Glycerine.
From www.youtube.com
Explaining Boiling Point (Simple Covalent, Intermolecular Forces) GCSE Chemistry Revision What Is The Boiling Point Glycerine Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: The boiling point of glycerin is 290°c. This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Thus ethylene glycol does not boil away when it is used as an antifreeze. Experimental densities at. What Is The Boiling Point Glycerine.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is The Boiling Point Glycerine The boiling point of glycerin is 290°c. Individual data points quantity value units. Learn about the structure, properties, and uses of glycerin here. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Thus ethylene glycol does not boil away when it is used as an antifreeze. This results in a. What Is The Boiling Point Glycerine.
From www.numerade.com
SOLVED 3.It is well known that thel boiling point of water 100" Celsius More Viscous, Or ticker What Is The Boiling Point Glycerine This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. The freezing points are reduced until glycerine concentration is. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Quantity value units method reference comment; Individual data points quantity value units. Learn about the structure,. What Is The Boiling Point Glycerine.
From www.mdpi.com
Processes Free FullText Pool Boiling Heat Transfer Coefficients in Mixtures of Water and What Is The Boiling Point Glycerine The boiling point of glycerin is 290°c. Individual data points quantity value units. Learn about the structure, properties, and uses of glycerin here. This results in a high boiling point—198°c; The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Experimental densities at pressures of up to 25 mpa, and. The. What Is The Boiling Point Glycerine.
From slideplayer.com
Phases of Matter Unit Notes ppt download What Is The Boiling Point Glycerine Thus ethylene glycol does not boil away when it is used as an antifreeze. The freezing points are reduced until glycerine concentration is. Learn about the structure, properties, and uses of glycerin here. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: Individual data points quantity value units. This. What Is The Boiling Point Glycerine.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Boiling Point Glycerine The freezing points are reduced until glycerine concentration is. The boiling point of glycerin is 290°c. This results in a high boiling point—198°c; Quantity value units method reference comment; Experimental densities at pressures of up to 25 mpa, and. Learn about the structure, properties, and uses of glycerin here. Individual data points quantity value units. The boiling points of glycerine. What Is The Boiling Point Glycerine.
From www.andreottiimpianti.com
glycerine distillation ANDREOTTI IMPIANTI What Is The Boiling Point Glycerine This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Experimental densities at pressures of up to 25 mpa, and. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: Individual data points quantity value units. Thus ethylene glycol does not boil away when. What Is The Boiling Point Glycerine.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Boiling Point Glycerine The boiling point of glycerin is 290°c. This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. Thus ethylene glycol does not boil away when it is used as an antifreeze. This results in a high boiling point—198°c; The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased. What Is The Boiling Point Glycerine.
From www.cosmacon.de
Glycerin Cosmacon What Is The Boiling Point Glycerine Quantity value units method reference comment; This results in a high boiling point—198°c; The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. Experimental densities at pressures of up to 25 mpa, and. Individual data points quantity value units. The boiling point of glycerin is 290°c. Learn about the structure, properties,. What Is The Boiling Point Glycerine.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols What Is The Boiling Point Glycerine Individual data points quantity value units. Thus ethylene glycol does not boil away when it is used as an antifreeze. This results in a high boiling point—198°c; This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. The boiling point of glycerin is 290°c. The boiling points of glycerine (also called glycerin or glycerol). What Is The Boiling Point Glycerine.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is The Boiling Point Glycerine Quantity value units method reference comment; This results in a high boiling point—198°c; Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: Learn about the structure, properties, and uses of glycerin here. Individual data points quantity value units. The boiling points of glycerine (also called glycerin or glycerol) water. What Is The Boiling Point Glycerine.
From www.worksheetsplanet.com
What is Boiling Point What Is The Boiling Point Glycerine Experimental densities at pressures of up to 25 mpa, and. Thus ethylene glycol does not boil away when it is used as an antifreeze. This high boiling point is a result of the strong hydrogen bonding between glycerin molecules, which. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. The. What Is The Boiling Point Glycerine.
From europepmc.org
Boiling points of the propylene glycol + glycerol system at 1 atmosphere pressure 188.6292 °C What Is The Boiling Point Glycerine This results in a high boiling point—198°c; Quantity value units method reference comment; The boiling point of glycerin is 290°c. Experimental densities at pressures of up to 25 mpa, and. Thus ethylene glycol does not boil away when it is used as an antifreeze. Individual data points quantity value units. Improving a variation of the dsc technique for measuring the. What Is The Boiling Point Glycerine.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Is The Boiling Point Glycerine The boiling point of glycerin is 290°c. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: Learn about the structure, properties, and uses of glycerin here. The freezing points are reduced until glycerine concentration is. Experimental densities at pressures of up to 25 mpa, and. This high boiling point. What Is The Boiling Point Glycerine.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is The Boiling Point Glycerine Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: The freezing points are reduced until glycerine concentration is. Experimental densities at pressures of up to 25 mpa, and. Learn about the structure, properties, and uses of glycerin here. Quantity value units method reference comment; The boiling points of glycerine. What Is The Boiling Point Glycerine.
From www.numerade.com
SOLVEDCalculate the freezing point and normal boiling point of each of the following solutions What Is The Boiling Point Glycerine This results in a high boiling point—198°c; Experimental densities at pressures of up to 25 mpa, and. Thus ethylene glycol does not boil away when it is used as an antifreeze. The boiling points of glycerine (also called glycerin or glycerol) water mixtures are reduced with increased amounts of glycerine. The freezing points are reduced until glycerine concentration is. Individual. What Is The Boiling Point Glycerine.
From www.numerade.com
SOLVED What is the boiling point of a solution of 1.0 g of glycerin, C3H5(OH)3, in 47.8 g of What Is The Boiling Point Glycerine Experimental densities at pressures of up to 25 mpa, and. Quantity value units method reference comment; Individual data points quantity value units. This results in a high boiling point—198°c; Thus ethylene glycol does not boil away when it is used as an antifreeze. The freezing points are reduced until glycerine concentration is. The boiling points of glycerine (also called glycerin. What Is The Boiling Point Glycerine.
From www.chegg.com
Solved 6. Consider the structures and boiling points for What Is The Boiling Point Glycerine Individual data points quantity value units. The freezing points are reduced until glycerine concentration is. Improving a variation of the dsc technique for measuring the boiling points of pure compounds at low pressures liquid glycerol: Experimental densities at pressures of up to 25 mpa, and. This high boiling point is a result of the strong hydrogen bonding between glycerin molecules,. What Is The Boiling Point Glycerine.