Making Dilutions Worksheet Answers . How much of it do you need to prepare 50 ml of a. A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Making dilutions worksheet remember that you can change the concentration of a solution by adding more solvent. I add 560 ml more water to it? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. There’s a bottle of 0.750 m nacl on a shelf. Perform the following dilutions calculation.
from www.docsity.com
Dilution m 1v 1=m 2v 2 1. How much of it do you need to prepare 50 ml of a. Perform the following dilutions calculation. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? There’s a bottle of 0.750 m nacl on a shelf. I add 560 ml more water to it?
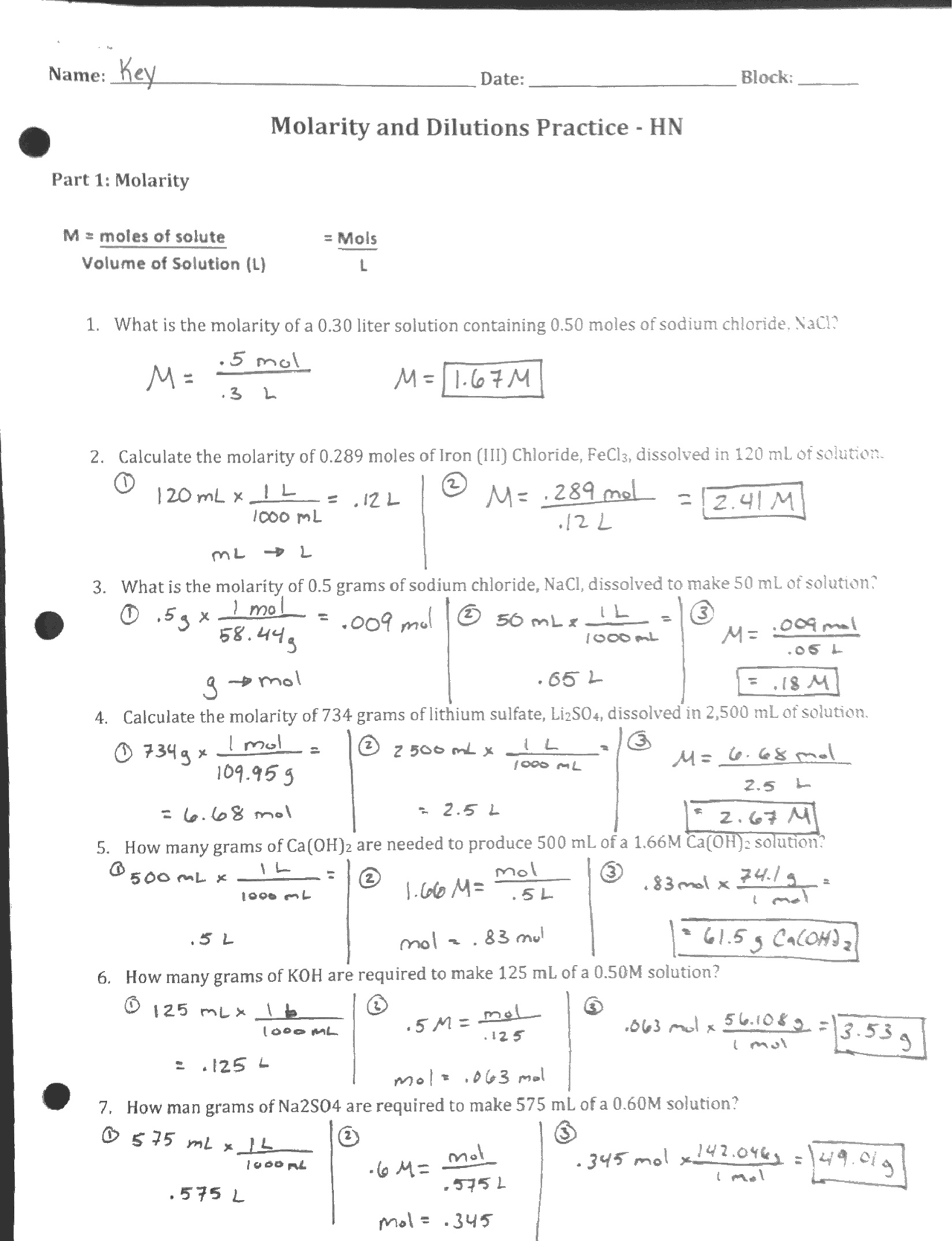
Molarity and Dilutions Practice Worksheet Key Docsity
Making Dilutions Worksheet Answers 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How much of it do you need to prepare 50 ml of a. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Making dilutions worksheet remember that you can change the concentration of a solution by adding more solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Perform the following dilutions calculation. I add 560 ml more water to it? There’s a bottle of 0.750 m nacl on a shelf.
From www.chegg.com
Solved Solutions and Dilutions. Worksheet 3. Here are some Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. I add 560 ml more water to it? Perform the following dilutions calculation. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. 1) if i add. Making Dilutions Worksheet Answers.
From www.docsity.com
Molarity and Dilutions Practice Worksheet Key Docsity Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Making dilutions worksheet remember that you can change the concentration of a solution by. Making Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Dilution SelfStudy Worksheet Dilutions 1 Spring 2020 Dilutions Making Dilutions Worksheet Answers There’s a bottle of 0.750 m nacl on a shelf. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. Making Dilutions Worksheet Answers.
From studylib.net
Solutions & Dilutions Worksheet Making Dilutions Worksheet Answers I add 560 ml more water to it? How much of it do you need to prepare 50 ml of a. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity. Making Dilutions Worksheet Answers.
From www.studocu.com
Making Dilutions Worksheet Name_________________________ Date Making Dilutions Worksheet Answers Making dilutions worksheet remember that you can change the concentration of a solution by adding more solvent. Dilution m 1v 1=m 2v 2 1. I add 560 ml more water to it? A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. How much of it do you need to prepare 50 ml of. Making Dilutions Worksheet Answers.
From www.worksheeto.com
7 Molarity Worksheet With Answers / Making Dilutions Worksheet Answers 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How much of it do you need to prepare 50 ml of a. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. A scientist has. Making Dilutions Worksheet Answers.
From www.chemistryworksheet.com
Dilution Calculation Worksheet With Answers Printable Pdf Download Making Dilutions Worksheet Answers A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what. Making Dilutions Worksheet Answers.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Making Dilutions Worksheet Answers I add 560 ml more water to it? Perform the following dilutions calculation. How much of it do you need to prepare 50 ml of a. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh. Making Dilutions Worksheet Answers.
From materialmagicsusana.z19.web.core.windows.net
Dilutions Worksheets Answers Making Dilutions Worksheet Answers I add 560 ml more water to it? Dilution m 1v 1=m 2v 2 1. Making dilutions worksheet remember that you can change the concentration of a solution by adding more solvent. Perform the following dilutions calculation. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. 1) if i. Making Dilutions Worksheet Answers.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. I add 560 ml more water to it? There’s a bottle of 0.750 m nacl on a shelf. 1) if. Making Dilutions Worksheet Answers.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to. Making Dilutions Worksheet Answers.
From lessonzonefassbinder.z19.web.core.windows.net
Making Dilutions Worksheets Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? How much of it do you need to prepare 50 ml of a. There’s. Making Dilutions Worksheet Answers.
From www.liveworksheets.com
Making Dilutions worksheet Live Worksheets Making Dilutions Worksheet Answers Perform the following dilutions calculation. Making dilutions worksheet remember that you can change the concentration of a solution by adding more solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. How much of it do you need to prepare 50 ml of. Making Dilutions Worksheet Answers.
From www.docsity.com
Making Dilutions Worksheet Answers Key Docsity Making Dilutions Worksheet Answers 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Perform the following dilutions calculation. Dilution m 1v 1=m 2v 2 1. A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. 1) if i add 25 ml of water to 125 ml of a 0.15. Making Dilutions Worksheet Answers.
From igneousrocksworksheetanswers.blogspot.com
Dilutions Worksheet Answers Maths Printables Free Making Dilutions Worksheet Answers 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. I add 560 ml more water to it? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. How much of it do you need to. Making Dilutions Worksheet Answers.
From www.formsbank.com
Top 10 Dilution Worksheet Templates free to download in PDF format Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. Perform the following dilutions calculation. I add 560 ml more water to it? 1) if i add 25. Making Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Solved Dilution Worksheet 2 Show all work in the spaces Making Dilutions Worksheet Answers A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. Perform the following dilutions calculation. There’s a bottle of 0.750 m nacl on a shelf. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. Making dilutions worksheet remember that you can change the. Making Dilutions Worksheet Answers.
From worksheets.decoomo.com
20++ Dilutions Worksheet Answers Worksheets Decoomo Making Dilutions Worksheet Answers There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2 1. Perform the following dilutions calculation. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i have 340 ml of a 0.5 m nabr solution,. Making Dilutions Worksheet Answers.
From answerlistsybil.z13.web.core.windows.net
Dilution Practice Problems Worksheet Answers Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. I add 560 ml more water to it? There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2 1. Making dilutions worksheet remember that you can change the. Making Dilutions Worksheet Answers.
From answerlistsybil.z13.web.core.windows.net
Dilution Practice Problems Worksheet Answers Making Dilutions Worksheet Answers How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. Making dilutions worksheet remember that you can change the concentration of a solution by adding more solvent. A scientist has a container with solid sodium hydroxide and a container of. Making Dilutions Worksheet Answers.
From igneousrocksworksheetanswers.blogspot.com
Dilutions Worksheet Answers Maths Printables Free Making Dilutions Worksheet Answers Perform the following dilutions calculation. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. How much of it do you need to prepare 50 ml of a.. Making Dilutions Worksheet Answers.
From lessonlistuncrushed.z22.web.core.windows.net
Making Dilutions Worksheets Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? How much of it do you need to prepare 50 ml of a. Perform the following dilutions calculation. A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium.. Making Dilutions Worksheet Answers.
From www.scribd.com
Making Dilutions Worksheet Download Free PDF Chemistry Physical Making Dilutions Worksheet Answers A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. I add 560 ml more water to it? Making dilutions worksheet remember that you can change the concentration of a solution by adding more solvent. There’s a bottle of 0.750 m nacl on a shelf. 1) if i add 25 ml of water to. Making Dilutions Worksheet Answers.
From www.formsbank.com
Dilution Worksheet With Answers printable pdf download Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. I add 560 ml more water to it? A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l. Making Dilutions Worksheet Answers.
From www.studocu.com
Dilution Worksheet Dilution Worksheet To make a dilution use the Making Dilutions Worksheet Answers A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. There’s a bottle of 0.750 m nacl on a shelf. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 1) if i add 25 ml of water. Making Dilutions Worksheet Answers.
From worksheets.clipart-library.com
Dilutions Worksheet Answers Printable Recipe Cards Central Making Dilutions Worksheet Answers How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Perform the following dilutions calculation. There’s a bottle of 0.750 m nacl on a. Making Dilutions Worksheet Answers.
From www.pdffiller.com
Fillable Online Making Dilutions Worksheet Key.pdf Fax Email Print Making Dilutions Worksheet Answers Making dilutions worksheet remember that you can change the concentration of a solution by adding more solvent. Perform the following dilutions calculation. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. 1) if i add 25 ml of water to 125 ml of a 0.15 m. Making Dilutions Worksheet Answers.
From lessondbjack.z13.web.core.windows.net
Dilutions Worksheet Chemistry Answers Making Dilutions Worksheet Answers Perform the following dilutions calculation. How much of it do you need to prepare 50 ml of a. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Dilution m 1v 1=m 2v 2 1. A scientist has a container with solid sodium hydroxide. Making Dilutions Worksheet Answers.
From kidsworksheetfun.com
Serial Dilutions Worksheet Answers Kidsworksheetfun Making Dilutions Worksheet Answers How much of it do you need to prepare 50 ml of a. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Dilution m 1v 1=m 2v 2 1. I add 560 ml more water to it? Making dilutions worksheet remember that you. Making Dilutions Worksheet Answers.
From lessonlistuncrushed.z22.web.core.windows.net
Making Dilutions Worksheets Making Dilutions Worksheet Answers Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Perform. Making Dilutions Worksheet Answers.
From materialmagicbayer.z19.web.core.windows.net
Making Dilutions Worksheets Making Dilutions Worksheet Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 1) if i have 340 ml of a. Making Dilutions Worksheet Answers.
From classschoolwinchell.z13.web.core.windows.net
Dilution Worksheet With Answers Making Dilutions Worksheet Answers A scientist has a container with solid sodium hydroxide and a container of 5.00 mol/l sodium. Dilution m 1v 1=m 2v 2 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution,. Making Dilutions Worksheet Answers.
From live.midifan.com
Making Dilutions Worksheet Making Dilutions Worksheet Answers I add 560 ml more water to it? There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. A scientist has a container with solid sodium hydroxide and a. Making Dilutions Worksheet Answers.
From aba.team
Making Dilutions worksheet Live Worksheets Dilutions Worksheet Making Dilutions Worksheet Answers 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. Perform the following dilutions calculation. I add 560 ml more water to it? 1) if i add 25 ml of water to. Making Dilutions Worksheet Answers.
From worksheets.decoomo.com
20++ Dilutions Worksheet Answers Worksheets Decoomo Making Dilutions Worksheet Answers Perform the following dilutions calculation. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need to prepare 50 ml of a. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution. Making Dilutions Worksheet Answers.