Titration With H2So4 . in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). Sulfuric acid, h 2 so 4 is a strong diprotic acid. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Sulfuric acid dissociates (ionises) in two stages:
from www.chegg.com
a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Sulfuric acid, h 2 so 4 is a strong diprotic acid. Sulfuric acid dissociates (ionises) in two stages: in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed.
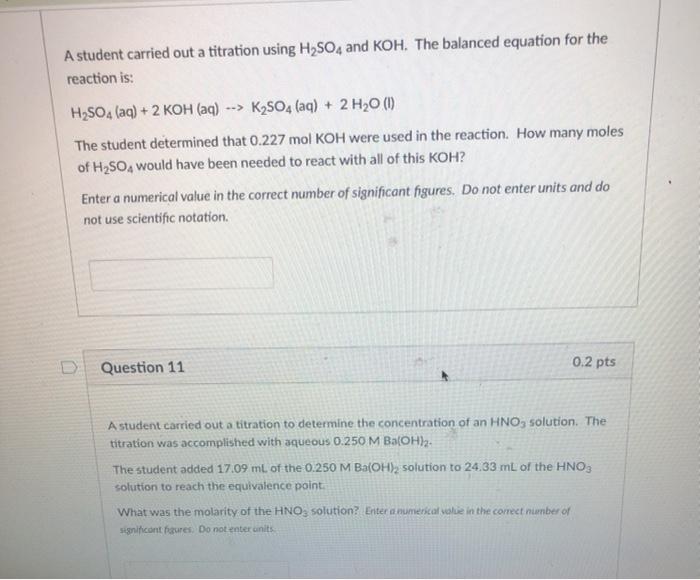
Solved A student carried out a titration using H2SO4 and
Titration With H2So4 in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). Sulfuric acid, h 2 so 4 is a strong diprotic acid. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. Sulfuric acid dissociates (ionises) in two stages: This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4).
From www.youtube.com
Molarity of H2SO4 Titration YouTube Titration With H2So4 a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. Sulfuric acid dissociates (ionises) in two stages: in this process, 2 moles (the. Titration With H2So4.
From www.coursehero.com
[Solved] A student performed three H2SO4 titrations in Part 2 using Titration With H2So4 in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Sulfuric acid dissociates (ionises) in two stages:. Titration With H2So4.
From www.chegg.com
Solved Titration of a 25.00 mL solution of H2SO4 requires Titration With H2So4 Sulfuric acid dissociates (ionises) in two stages: Sulfuric acid, h 2 so 4 is a strong diprotic acid. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium. Titration With H2So4.
From www.coursehero.com
[Solved] A student performed three H2SO4 titrations in Part 2 using Titration With H2So4 in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. Sulfuric acid dissociates (ionises) in two stages: This results in the formation of two moles of water (h. Titration With H2So4.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Titration With H2So4 a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. This results in the formation of. Titration With H2So4.
From www.chegg.com
Solved H2SO4 Titration H2S0,(aq) + 2NaOH(aq) Na,S04(aq) + Titration With H2So4 Sulfuric acid, h 2 so 4 is a strong diprotic acid. a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh). Titration With H2So4.
From www.youtube.com
CHEM 1111 Lab 7 Determination of the Concentration of H2SO4 by Titration With H2So4 Sulfuric acid dissociates (ionises) in two stages: a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a. Titration With H2So4.
From www.coursehero.com
[Solved] A 50.00 mL sample of an aqueous H2SO4 solution was titrated Titration With H2So4 Sulfuric acid, h 2 so 4 is a strong diprotic acid. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). in a titration of sulfuric acid against sodium hydroxide,. Titration With H2So4.
From www.youtube.com
Titration Curve H2SO4 + NaOH YouTube Titration With H2So4 a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. in this process, 2 moles (the molecular weight of a substance expressed in. Titration With H2So4.
From exoqwdiya.blob.core.windows.net
H2So4 Titration Graph at Jewell Hamilton blog Titration With H2So4 Sulfuric acid, h 2 so 4 is a strong diprotic acid. Sulfuric acid dissociates (ionises) in two stages: a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \:. Titration With H2So4.
From www.youtube.com
The titration of 25 0 mL of an unknown concentration of H2SO4 solution Titration With H2So4 in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). . Titration With H2So4.
From www.chegg.com
Solved 25.00mL of H2SO4 was titrated with 0.15M NaOH. Titration With H2So4 a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. in a titration of sulfuric. Titration With H2So4.
From www.researchgate.net
Plots obtained during titration of 20.00 mL H2SO4 0.024 M + 20.0 mL Titration With H2So4 a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. Sulfuric acid,. Titration With H2So4.
From www.youtube.com
Titration Reaction H2SO4 and LiOH YouTube Titration With H2So4 a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. Sulfuric acid dissociates (ionises) in two stages: Sulfuric acid, h 2 so 4 is a strong diprotic acid. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the. Titration With H2So4.
From exoqwdiya.blob.core.windows.net
H2So4 Titration Graph at Jewell Hamilton blog Titration With H2So4 in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. Sulfuric acid, h 2 so 4 is a strong diprotic acid. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3. Titration With H2So4.
From studylib.net
Conductivity Titration of a Ba(OH)2 solution with H2SO4 Titration With H2So4 a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Sulfuric acid, h 2 so 4 is a strong diprotic acid. in a titration of sulfuric acid against sodium. Titration With H2So4.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Titration With H2So4 in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Sulfuric acid, h 2 so 4 is a strong diprotic acid. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Sulfuric acid dissociates (ionises) in two stages: This results in the formation of. Titration With H2So4.
From www.studypool.com
SOLUTION The aim is to find the accurate concentration of sulphuric Titration With H2So4 a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Sulfuric acid dissociates (ionises) in two stages: in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a. Titration With H2So4.
From www.scribd.com
Acid Base Titration Lab H2so4 + Naoh AP Chem 2 Titration Chemistry Titration With H2So4 This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. Sulfuric acid dissociates (ionises) in two stages:. Titration With H2So4.
From dxoejubzg.blob.core.windows.net
Titration H2So4 at Gallo blog Titration With H2So4 a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Sulfuric acid, h 2 so 4. Titration With H2So4.
From dxoejubzg.blob.core.windows.net
Titration H2So4 at Gallo blog Titration With H2So4 a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. Sulfuric acid dissociates (ionises) in two stages: This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). in this process, 2 moles (the molecular weight of a substance expressed in. Titration With H2So4.
From www.numerade.com
SOLVED During a titration, 10.00 mL of sulphuric acid, H2SO4 is Titration With H2So4 a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). a titration is a process in which a measured amount of a solution is reacted with a known volume of. Titration With H2So4.
From www.numerade.com
SOLVED For the titration reaction below H2SO4 + 2NaOH > 2H2O Titration With H2So4 Sulfuric acid, h 2 so 4 is a strong diprotic acid. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). in a titration of sulfuric acid against sodium hydroxide,. Titration With H2So4.
From www.chegg.com
Solved 25.00mL of H2SO4 was titrated with 0.15M NaOH. Titration With H2So4 in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). Sulfuric acid, h 2 so 4 is a strong diprotic acid. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate. Titration With H2So4.
From www.numerade.com
A student carried out a titration using H2SO4 and KOH. The balanced Titration With H2So4 Sulfuric acid dissociates (ionises) in two stages: a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. Sulfuric acid, h 2 so 4 is. Titration With H2So4.
From www.numerade.com
SOLVED A solution of NaOH is titrated with H2SO4. It is found that 20. Titration With H2So4 in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric. Titration With H2So4.
From www.chegg.com
Solved An unknown solution of H2SO4 is titrated with NaOH. Titration With H2So4 Sulfuric acid dissociates (ionises) in two stages: in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Sulfuric acid, h 2 so 4 is a strong diprotic acid. in a titration, 25.00 cm 3. Titration With H2So4.
From www.chegg.com
Solved A student carried out a titration using H2SO4 and Titration With H2So4 in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Sulfuric acid dissociates (ionises) in two stages: Sulfuric acid, h 2 so 4. Titration With H2So4.
From www.scribd.com
Conductometric Titration of H2SO4 and HCl Acid Titration Titration With H2So4 in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. Sulfuric acid, h 2 so 4 is a strong diprotic acid. a titration is a process in which a. Titration With H2So4.
From www.numerade.com
SOLVED Determination of concentration of H2SO4 Employing acidbase Titration With H2So4 in this process, 2 moles (the molecular weight of a substance expressed in grams) of sodium hydroxide (naoh) combine with one mole of sulfuric acid (h 2 so 4). in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration is a process in which a measured amount of a solution is reacted with a. Titration With H2So4.
From www.researchgate.net
Titration of 1 mL diluted bath (H2SO4/H3PO4) with 0.5 M NaOH in water Titration With H2So4 Sulfuric acid, h 2 so 4 is a strong diprotic acid. a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: in a titration, 25.00 cm 3. Titration With H2So4.
From mavink.com
H2so4 Titration Curve Titration With H2So4 in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). a titration is a process in which a measured amount of a. Titration With H2So4.
From www.numerade.com
SOLVED Given the balanced equation H2SO4 + Na2CO3 > Na2SO4 + H2O Titration With H2So4 a titration is a process in which a measured amount of a solution is reacted with a known volume of another solution (one of the solutions has an unknown. This results in the formation of two moles of water (h 2 o) and one mole of sodium sulfate (na 2 so 4). Sulfuric acid, h 2 so 4 is. Titration With H2So4.
From www.coursehero.com
[Solved] For the titration of sulfuric acid (H2SO4) with sodium hyd Titration With H2So4 in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. This results in the formation of two moles. Titration With H2So4.
From mavink.com
H2so4 Titration Curve Titration With H2So4 Sulfuric acid, h 2 so 4 is a strong diprotic acid. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration using $\ce{naoh}$ (the same $\ce{naoh}$ as used in the previous section) was performed. Sulfuric acid dissociates (ionises) in two stages: in. Titration With H2So4.