Copper Wire Silver Nitrate Redox Reaction . The reaction in a test tube. The solution gradually acquires the blue color. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. The reaction between copper and silver nitrate solution. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. Within minutes, the wire begins to look fuzzy or furry as small silver. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of.
from socratic.org
The solution gradually acquires the blue color. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The reaction in a test tube. The reaction between copper and silver nitrate solution. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. Within minutes, the wire begins to look fuzzy or furry as small silver. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of.
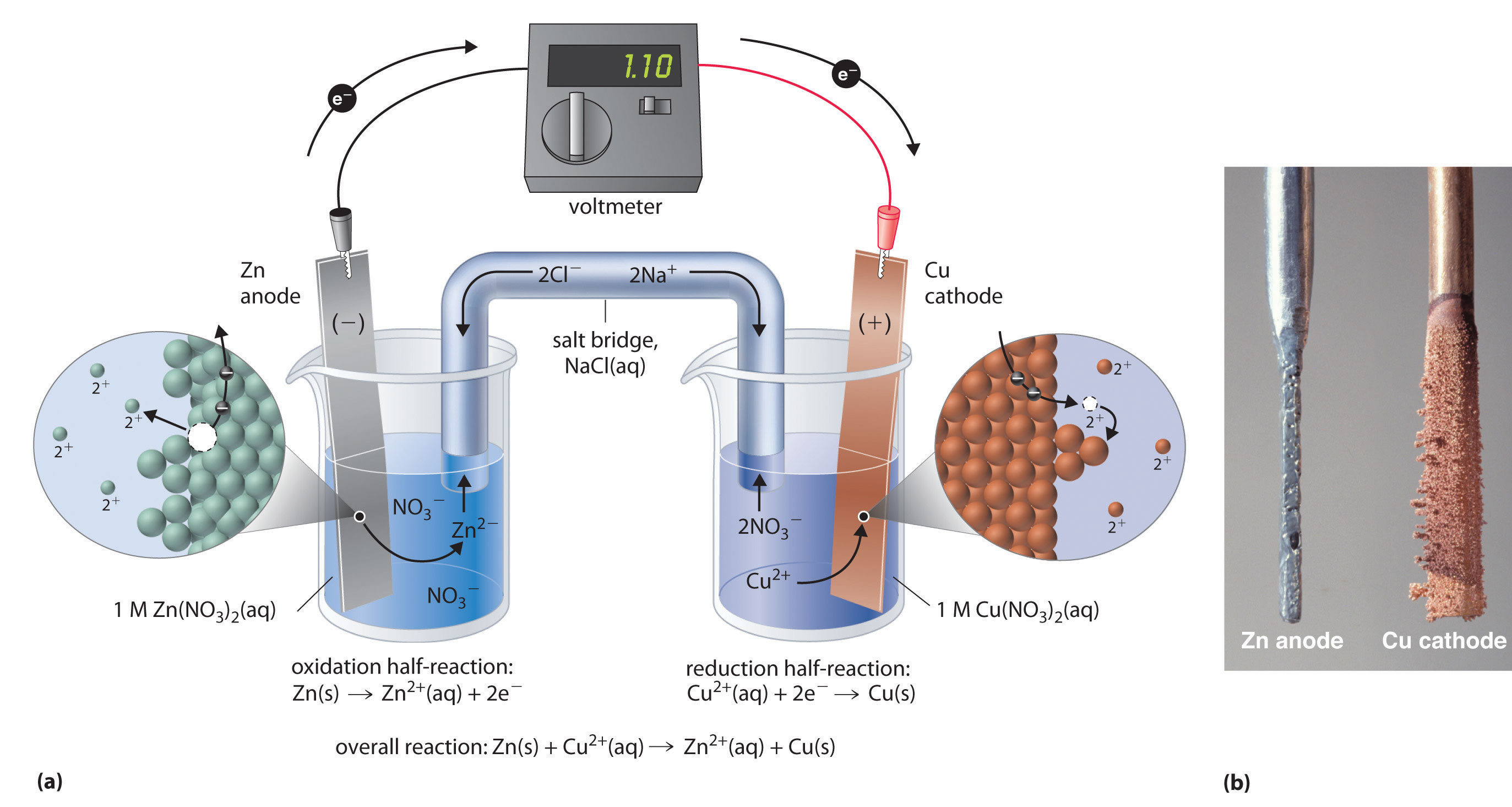
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode
Copper Wire Silver Nitrate Redox Reaction Within minutes, the wire begins to look fuzzy or furry as small silver. The reaction between copper and silver nitrate solution. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. The reaction in a test tube. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Within minutes, the wire begins to look fuzzy or furry as small silver. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. The solution gradually acquires the blue color. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of.
From lillyfersmcknight.blogspot.com
Copper Silver Nitrate Yields Copper Ii Nitrate Silver Copper Wire Silver Nitrate Redox Reaction Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece. Copper Wire Silver Nitrate Redox Reaction.
From www.sciencephoto.com
Copper Reacts with Silver Nitrate, 4 of 6 Stock Image C028/1082 Copper Wire Silver Nitrate Redox Reaction The solution gradually acquires the blue color. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. This video captures a demonstration of a. Copper Wire Silver Nitrate Redox Reaction.
From fphoto.photoshelter.com
science chemical reaction redox silver copper Fundamental Photographs Copper Wire Silver Nitrate Redox Reaction The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. Within minutes, the wire begins to look fuzzy or furry as small silver. An alternative way to demonstrate this displacement. Copper Wire Silver Nitrate Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction silver nitrate copper Fundamental Copper Wire Silver Nitrate Redox Reaction Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate.. Copper Wire Silver Nitrate Redox Reaction.
From www.slideserve.com
PPT OxidationReduction Reactions & Electrochemistry PowerPoint Copper Wire Silver Nitrate Redox Reaction Within minutes, the wire begins to look fuzzy or furry as small silver. The solution gradually acquires the blue color. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. The reaction between copper and silver nitrate solution. Cu + agno3 = cu(no3)2 + ag is a single displacement. Copper Wire Silver Nitrate Redox Reaction.
From www.youtube.com
Redox Reaction Holiday ChemisTree! Copper + Silver Nitrate (Holiday Copper Wire Silver Nitrate Redox Reaction An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The. Copper Wire Silver Nitrate Redox Reaction.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Wire Silver Nitrate Redox Reaction A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in. Copper Wire Silver Nitrate Redox Reaction.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Wire Silver Nitrate Redox Reaction The reaction in a test tube. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. The reaction between copper and silver nitrate solution. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. The solution gradually. Copper Wire Silver Nitrate Redox Reaction.
From www.numerade.com
SOLVED The following diagram shows the electrochemical cell with redox Copper Wire Silver Nitrate Redox Reaction Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. The reaction of copper wire with silver nitrate in aqueous. Copper Wire Silver Nitrate Redox Reaction.
From webmis.highland.cc.il.us
OxidationReduction Reactions in Solution Copper Wire Silver Nitrate Redox Reaction A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. This video captures a demonstration of a reaction of copper wire with a solution. Copper Wire Silver Nitrate Redox Reaction.
From fyohrrkto.blob.core.windows.net
Copper Wire And Silver Nitrate Experiment at Janie Parker blog Copper Wire Silver Nitrate Redox Reaction The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Within minutes, the wire begins to look fuzzy or furry as small silver. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. Cu + agno3 = cu(no3)2 +. Copper Wire Silver Nitrate Redox Reaction.
From www.reddit.com
Copper wire undergoing a redox/displacement reaction with a silver Copper Wire Silver Nitrate Redox Reaction This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. Within minutes, the wire begins to look fuzzy or furry as small silver. Cu + agno3 = cu(no3)2 + ag is a single displacement. Copper Wire Silver Nitrate Redox Reaction.
From www.youtube.com
Redox Reaction Copper and Silver Nitrate YouTube Copper Wire Silver Nitrate Redox Reaction The solution gradually acquires the blue color. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. The reaction between copper and silver nitrate solution. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. An alternative way to demonstrate. Copper Wire Silver Nitrate Redox Reaction.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Copper Wire Silver Nitrate Redox Reaction The reaction between copper and silver nitrate solution. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. A simple redox reaction occurs when copper. Copper Wire Silver Nitrate Redox Reaction.
From www.copperwiresuppliers.net
Copper Wire Silver Nitrate Copper Wire SuppliersCopper Wire Suppliers Copper Wire Silver Nitrate Redox Reaction The reaction in a test tube. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. If you hang a. Copper Wire Silver Nitrate Redox Reaction.
From www.doubtnut.com
When a copper rod is dipped in aqueous silver nitrate solution , the c Copper Wire Silver Nitrate Redox Reaction Within minutes, the wire begins to look fuzzy or furry as small silver. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. An alternative way to demonstrate this displacement reaction is to drop a 5 cm. Copper Wire Silver Nitrate Redox Reaction.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Copper Wire Silver Nitrate Redox Reaction Within minutes, the wire begins to look fuzzy or furry as small silver. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. If you hang a coil of copper wire in. Copper Wire Silver Nitrate Redox Reaction.
From www.youtube.com
Copper Penny Reaction With Silver Nitrate (Cu + AgNO3) YouTube Copper Wire Silver Nitrate Redox Reaction Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper. Copper Wire Silver Nitrate Redox Reaction.
From www.thesciencehive.co.uk
Redox and Electrode Potentials* — the science sauce Copper Wire Silver Nitrate Redox Reaction The solution gradually acquires the blue color. Within minutes, the wire begins to look fuzzy or furry as small silver. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. The reaction between copper and silver nitrate solution. The reaction in a test tube. This video. Copper Wire Silver Nitrate Redox Reaction.
From www.facebook.com
Copper wire undergoing a redox displacement reaction with a silver Copper Wire Silver Nitrate Redox Reaction Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution.. Copper Wire Silver Nitrate Redox Reaction.
From www.youtube.com
Balance AgNO3 + Cu = Cu(NO3)2 + Ag (Silver Nitrate and Copper) YouTube Copper Wire Silver Nitrate Redox Reaction An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver. Copper Wire Silver Nitrate Redox Reaction.
From www.slideserve.com
PPT Preparation of silver nitrate and its uses PowerPoint Copper Wire Silver Nitrate Redox Reaction The solution gradually acquires the blue color. The reaction between copper and silver nitrate solution. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. The reaction in a test tube. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire. Copper Wire Silver Nitrate Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction silver nitrate copper Fundamental Copper Wire Silver Nitrate Redox Reaction An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. The reaction in a test tube. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. Cu + agno3 = cu(no3)2 + ag is a. Copper Wire Silver Nitrate Redox Reaction.
From 9gag.com
Copper wire undergoing a redox/displacement reaction with a silver Copper Wire Silver Nitrate Redox Reaction The reaction between copper and silver nitrate solution. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. A simple demonstration of a redox reaction involves placing a. Copper Wire Silver Nitrate Redox Reaction.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Copper Wire Silver Nitrate Redox Reaction If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. Within minutes, the wire begins to look fuzzy or furry as small silver. The reaction. Copper Wire Silver Nitrate Redox Reaction.
From slideplayer.com
Chemistry 30 Unit 7 REDOX Reactions. ppt download Copper Wire Silver Nitrate Redox Reaction This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. Within minutes, the wire begins to look fuzzy or furry as small silver. If you hang a coil of. Copper Wire Silver Nitrate Redox Reaction.
From byjus.com
A student performs an experiment in which he dipped a copper coil to Copper Wire Silver Nitrate Redox Reaction A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the. Copper Wire Silver Nitrate Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Copper Wire Silver Nitrate Redox Reaction The reaction in a test tube. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The reaction between copper and silver nitrate solution. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. This video captures a demonstration of a reaction of. Copper Wire Silver Nitrate Redox Reaction.
From brainly.in
why the solution of silver nitrate blue in colour after Copper Wire Silver Nitrate Redox Reaction The solution gradually acquires the blue color. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. The reaction between copper and silver nitrate solution. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. A simple redox reaction occurs. Copper Wire Silver Nitrate Redox Reaction.
From www.numerade.com
The reaction between solid copper and aqueous silver nitrate produces Copper Wire Silver Nitrate Redox Reaction A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. An alternative way to demonstrate this displacement. Copper Wire Silver Nitrate Redox Reaction.
From www.sciencephoto.com
Silver nitratecopper displacement reaction Stock Image C002/7894 Copper Wire Silver Nitrate Redox Reaction The reaction in a test tube. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. If you hang a coil of copper wire in some colourless silver nitrate solution, the copper gets covered in. The solution gradually acquires the blue color. A simple. Copper Wire Silver Nitrate Redox Reaction.
From www.thesciencehive.co.uk
Redox and Electrode Potentials* — the science sauce Copper Wire Silver Nitrate Redox Reaction The reaction between copper and silver nitrate solution. Cu + agno3 = cu(no3)2 + ag is a single displacement (substitution) reaction where one mole of solid copper [cu] and two moles of. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. The reaction of copper wire with silver nitrate. Copper Wire Silver Nitrate Redox Reaction.
From lillyfersmcknight.blogspot.com
Copper Silver Nitrate Yields Copper Ii Nitrate Silver Copper Wire Silver Nitrate Redox Reaction This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. The solution. Copper Wire Silver Nitrate Redox Reaction.
From www.alamy.com
Metal displacement reaction. The solution of silver nitrate (AgNO3 Copper Wire Silver Nitrate Redox Reaction Within minutes, the wire begins to look fuzzy or furry as small silver. An alternative way to demonstrate this displacement reaction is to drop a 5 cm piece of 0.5 mm copper wire into a 8 cm depth of. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire.. Copper Wire Silver Nitrate Redox Reaction.
From slideplayer.com
Introduction to Redox Mrs. Kay Chemistry 12 Chapter 18 Pages ppt download Copper Wire Silver Nitrate Redox Reaction The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Within minutes, the wire begins to look fuzzy or furry as small silver. The reaction in a test tube. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution.. Copper Wire Silver Nitrate Redox Reaction.