Calorimeter Method Definition . For example, when an exothermic reaction. For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from www.learnable.education
calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
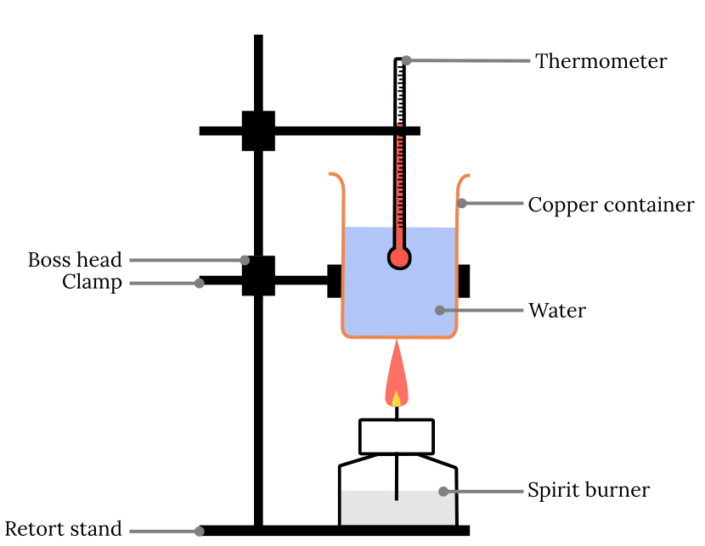
Year 11 Chemistry Practical Investigation Calorimetry Experiment
Calorimeter Method Definition calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer Simulation CIDER Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used to measure the amount of heat involved in a. Calorimeter Method Definition.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types, Examples and Uses CollegeSearch Calorimeter Method Definition calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in. Calorimeter Method Definition.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in. Calorimeter Method Definition.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device. Calorimeter Method Definition.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimeter Method Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such. Calorimeter Method Definition.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Method Definition For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a method of measuring the heat transfer within a chemical reaction or. Calorimeter Method Definition.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? Calorimeter Method Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as. Calorimeter Method Definition.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Method Definition calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used. Calorimeter Method Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimeter Method Definition.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Calorimeter Method Definition For example, when an exothermic reaction. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter is a. Calorimeter Method Definition.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Calorimeter Method Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used for calorimetry, or the process of measuring the heat of. Calorimeter Method Definition.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeters Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. . Calorimeter Method Definition.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimeter Method Definition For example, when an exothermic reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Method Definition.
From www.indirectcalorimetry.net
Understanding Indirect Calorimetry Indirect Calorimetry Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the. Calorimeter Method Definition.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimeter Method Definition.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Method Definition calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a. Calorimeter Method Definition.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimeter Method Definition.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Method Definition calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a. Calorimeter Method Definition.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimeter Method Definition calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. For example, when an exothermic reaction. a calorimeter is a. Calorimeter Method Definition.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Engineering Learn Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Calorimeter Method Definition.
From olivelmartino.blob.core.windows.net
Energy Calorimetry Definition at olivelmartino blog Calorimeter Method Definition calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount. Calorimeter Method Definition.
From www.researchgate.net
A schematic of the calorimeterbased method for the thermal... Download Scientific Diagram Calorimeter Method Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to. Calorimeter Method Definition.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Method Definition For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used for calorimetry, or the process of measuring. Calorimeter Method Definition.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Method Definition.
From users.highland.edu
Calorimetry Calorimeter Method Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter is a device used for calorimetry, or the process of measuring. Calorimeter Method Definition.
From kaffee.50webs.com
Lab Calorimetry Calorimeter Method Definition calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical. Calorimeter Method Definition.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Method Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction. a calorimeter is a device used for calorimetry, or the process of. Calorimeter Method Definition.
From studylib.net
Calorimetry Calorimeter Method Definition calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry. Calorimeter Method Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Method Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions. Calorimeter Method Definition.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Calorimeter Method Definition For example, when an exothermic reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring. Calorimeter Method Definition.
From exoarnnpu.blob.core.windows.net
Calorimeter Description And Function at Kyla Ochs blog Calorimeter Method Definition calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter Method Definition.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Method Definition For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used for calorimetry, or the process of measuring. Calorimeter Method Definition.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimeter Method Definition For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used for calorimetry, or the process of. Calorimeter Method Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Method Definition a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between. For example, when an exothermic reaction. For example, when an exothermic reaction. a calorimeter is. Calorimeter Method Definition.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimeter Method Definition For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. calorimetry is a method of measuring the heat transfer within a chemical. Calorimeter Method Definition.