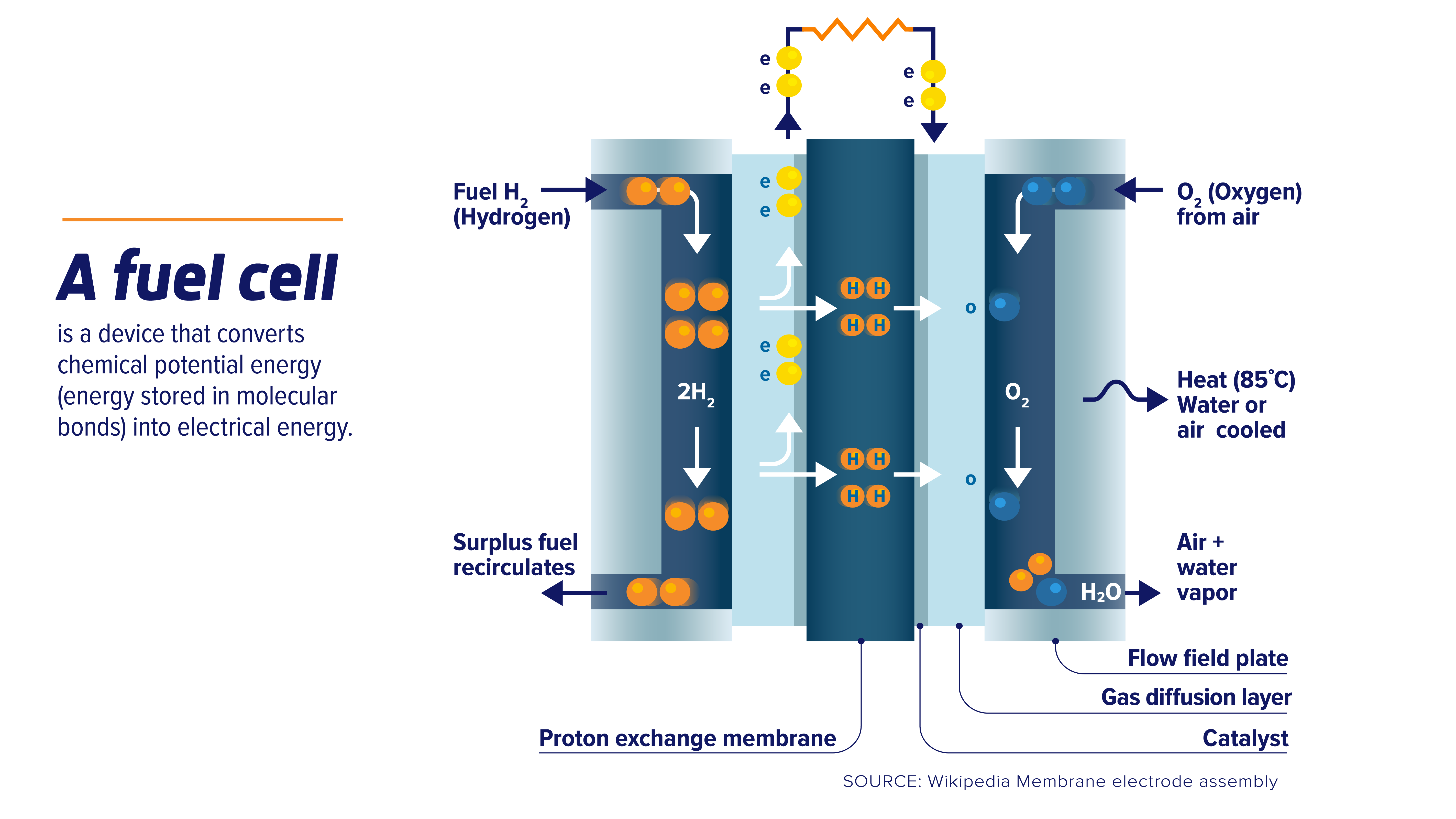
Fuel Cells Revision . fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. here we learn to evaluate the use of hydrogen fuel cells in comparison. The fuel is added to the cell and then there is. how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with.
from www.chfca.ca
fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. The fuel is added to the cell and then there is. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. here we learn to evaluate the use of hydrogen fuel cells in comparison.
About Fuel Cells CHFCA
Fuel Cells Revision a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. The fuel is added to the cell and then there is. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. here we learn to evaluate the use of hydrogen fuel cells in comparison.
From www.tes.com
Fuel Cells Home Learning Worksheet GCSE Teaching Resources Fuel Cells Revision a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by. Fuel Cells Revision.
From www.savemyexams.com
Fuel Cells HL IB Chemistry Revision Notes 2025 Save My Exams Fuel Cells Revision fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The fuel is added to the cell and then there is. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. how fuel cells work,. Fuel Cells Revision.
From www.fuelcellenergy.com
Benefits of Fuel Cells in Energy Efficient Power Generation Fuel Cells Revision how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. here we learn to evaluate the use of hydrogen fuel cells in. Fuel Cells Revision.
From www.dataphysics-instruments.com
How measuring contact angles at high temperatures can help develop fuel cells DataPhysics Fuel Cells Revision a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. fuel cells close fuel cell device that produces. Fuel Cells Revision.
From www.science-revision.co.uk
Fuel Cells Fuel Cells Revision fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. here we learn to evaluate the use of hydrogen fuel cells in comparison. The fuel is added to the cell and then. Fuel Cells Revision.
From www.researchgate.net
Classification of fuel cells. Download Scientific Diagram Fuel Cells Revision fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. The fuel is added to the cell and then there is. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. fuel cells produce. Fuel Cells Revision.
From lylagokejames.blogspot.com
Describe How a Fuel Cell Works Fuel Cells Revision a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such. Fuel Cells Revision.
From powerup-tech.com
The ABC of Fuel Cells PowerUP Energy Technologies Fuel Cells Revision a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. The fuel is added to the cell and then. Fuel Cells Revision.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cells Revision fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. The fuel is added to the cell and then there is. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. here we learn to evaluate the use of hydrogen fuel cells. Fuel Cells Revision.
From cmsw.mit.edu
» Small Steps and Giant Leaps in Fuel Cell Technologies Angles / 2022 Fuel Cells Revision fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. The fuel is added to the cell and then there is. here we learn to evaluate the use of hydrogen fuel cells in comparison. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry. Fuel Cells Revision.
From www.comsol.com
4 Examples of Fuel Cell Modeling in COMSOL Multiphysics® COMSOL Blog Fuel Cells Revision how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. The fuel is added to the cell and then there is. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. fuel cells produce electrical energy using a reaction. Fuel Cells Revision.
From www.elomatic.com
Fuel cells Elomatic Fuel Cells Revision a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. The fuel is added to the cell and then there is. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. fuel. Fuel Cells Revision.
From encyclopedia.pub
Fuel Cell HeavyDuty Vehicle Encyclopedia MDPI Fuel Cells Revision fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. The fuel is added to the cell and then there is. a fuel cell like this will continue to. Fuel Cells Revision.
From siqens.de
Methanol fuel cell Working principle and different types SIQENS Fuel Cells Revision here we learn to evaluate the use of hydrogen fuel cells in comparison. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. . Fuel Cells Revision.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cells Revision fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of. Fuel Cells Revision.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Fuel Cells Revision here we learn to evaluate the use of hydrogen fuel cells in comparison. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available.. Fuel Cells Revision.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cells Revision a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. The fuel is added to the cell and then there is. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. how fuel cells work,. Fuel Cells Revision.
From www.lean-cat.com
Fuel Cells › Fuel Cells Revision The fuel is added to the cell and then there is. here we learn to evaluate the use of hydrogen fuel cells in comparison. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells produce electrical energy using a reaction between. Fuel Cells Revision.
From 3dprint.com
Researchers at Northwestern University Develop Material to 3D Print Fuel Cells Fuel Cells Revision here we learn to evaluate the use of hydrogen fuel cells in comparison. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. a fuel cell is an electrochemical cell that. Fuel Cells Revision.
From semiengineering.com
Fuel Cells And The IoE Fuel Cells Revision a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. The fuel is added to the cell and then there is. here we learn to evaluate the use of hydrogen fuel cells in comparison. fuel cells close fuel cell device that produces. Fuel Cells Revision.
From onlinelibrary.wiley.com
Fuel Cells Wiley Online Library Fuel Cells Revision here we learn to evaluate the use of hydrogen fuel cells in comparison. The fuel is added to the cell and then there is. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel. Fuel Cells Revision.
From studylib.net
Activity 1.3.1 Fuel Cell User Guide Fuel Cells Revision The fuel is added to the cell and then there is. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. here we learn to evaluate. Fuel Cells Revision.
From www.researchgate.net
Schematic of 61a direct methanol fuel cell (DMFC)... Download Scientific Diagram Fuel Cells Revision a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. The fuel is added to the cell and then there is. fuel cells. Fuel Cells Revision.
From chemistry.stackexchange.com
electrochemistry Electrode polarity in fuel cells Chemistry Stack Exchange Fuel Cells Revision a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. fuel cells close fuel cell device that produces a voltage continuously when supplied. Fuel Cells Revision.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink Fuel Cells Revision a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. a fuel cell like this will continue to operate and produce electrical. Fuel Cells Revision.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Engineeringity Fuel Cells Revision here we learn to evaluate the use of hydrogen fuel cells in comparison. The fuel is added to the cell and then there is. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuel cells close fuel cell device that produces a. Fuel Cells Revision.
From www.pinterest.com
ENERGY CHANGES FUEL CELLS ADVANTAGES AND DISADVANTAGES REVISION SUMMARY AND EXAM Fuel Cells Revision fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. here we learn to evaluate the use of hydrogen fuel cells in comparison. revision notes. Fuel Cells Revision.
From www.researchgate.net
Overview of the various types of fuel cell technology. Typically used... Download Scientific Fuel Cells Revision a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. fuel cells produce electrical energy using a reaction between an external fuel source. Fuel Cells Revision.
From www.intelligent-energy.com
Fuel Cell FAQs Intelligent Energy Fuel Cells Revision revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. fuel cells produce electrical energy using a reaction between an external fuel. Fuel Cells Revision.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Fuel Cells Revision fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. revision. Fuel Cells Revision.
From www.youtube.com
Comparison of Fuel Cells YouTube Fuel Cells Revision fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the. Fuel Cells Revision.
From webprod.ki.si
GrapheneDerived Carbon Support Boosts Proton Exchange Membrane Fuel Cell Catalyst Stability Fuel Cells Revision here we learn to evaluate the use of hydrogen fuel cells in comparison. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. a fuel cell is an electrochemical. Fuel Cells Revision.
From www.slideserve.com
PPT Fuel Cell PowerPoint Presentation, free download ID491999 Fuel Cells Revision how fuel cells work, equations for the anode and cathode reactions in a hydrogen fuel cells, advantages and disadvantages of. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. fuel cells produce electrical energy using a reaction between an external fuel source (often hydrogen) and oxygen. here. Fuel Cells Revision.
From www.ameteksurfacevision.com
Batteries Fuel Cells Batteries AMETEK Surface Vision Fuel Cells Revision fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. a fuel cell is an electrochemical cell that converts the chemical energy of a fuel, such as hydrogen, into electrical energy by reacting it with. here we learn to evaluate the use of hydrogen fuel cells in comparison. . Fuel Cells Revision.
From scholarsharbor.com
Fuel Cells Scholars Harbor Fuel Cells Revision revision notes on fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. The fuel is added to the cell and then there is. fuel cells close fuel cell device that produces a voltage continuously when supplied with a fuel and oxygen. a fuel cell is an electrochemical cell that. Fuel Cells Revision.