Buffer Solution Questions And Answers . systematic solution to buffer problems; Calculate the ph of an unbuffered 0.010m acetic acid solution. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. Here, we have a few questions with answers based on the buffer. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. Representing buffer solutions with ladder diagrams; how good are you at solving buffer solution practice problems? the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps.
from www.chegg.com
a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. Representing buffer solutions with ladder diagrams; Here, we have a few questions with answers based on the buffer. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. Calculate the ph of an unbuffered 0.010m acetic acid solution. how good are you at solving buffer solution practice problems? systematic solution to buffer problems;
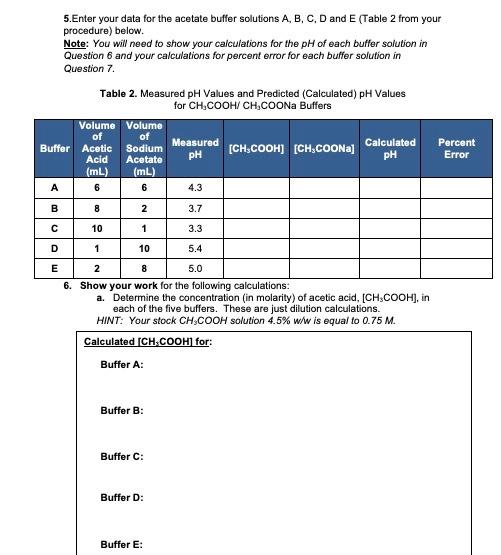
Solved 5.Enter your data for the acetate buffer solutions A,
Buffer Solution Questions And Answers the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. Representing buffer solutions with ladder diagrams; how good are you at solving buffer solution practice problems? systematic solution to buffer problems; the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. Calculate the ph of an unbuffered 0.010m acetic acid solution. Here, we have a few questions with answers based on the buffer.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide Buffer Solution Questions And Answers Calculate the ph of an unbuffered 0.010m acetic acid solution. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. systematic solution to buffer problems; Here, we have a few questions with answers based on the buffer.. Buffer Solution Questions And Answers.
From www.numerade.com
SOLVED Buffers Observing the Effect of Adding Acid or Base In this Buffer Solution Questions And Answers Calculate the ph of an unbuffered 0.010m acetic acid solution. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions.. Buffer Solution Questions And Answers.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Questions And Answers Representing buffer solutions with ladder diagrams; Here, we have a few questions with answers based on the buffer. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. Calculate the ph of an unbuffered 0.010m acetic acid solution. systematic solution to buffer problems; test your knowledge on. Buffer Solution Questions And Answers.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Questions And Answers a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. test your knowledge on buffer solutions. Buffer Solution Questions And Answers.
From studylib.net
Chemguide questions BUFFER SOLUTIONS Buffer Solution Questions And Answers Here, we have a few questions with answers based on the buffer. how good are you at solving buffer solution practice problems? a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. systematic solution to buffer. Buffer Solution Questions And Answers.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Questions And Answers test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. how good are you at solving buffer solution practice problems? Here, we have a few questions with answers based on the buffer. Representing buffer solutions with ladder diagrams; Calculate the ph of an unbuffered 0.010m acetic acid solution. the ability. Buffer Solution Questions And Answers.
From www.scribd.com
AP Unit9 Worksheet Answers Buffer Solution Acid Buffer Solution Questions And Answers the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. Representing buffer solutions with ladder diagrams; test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3. Buffer Solution Questions And Answers.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Questions And Answers the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. Here, we have a few questions with answers based on the buffer. Representing buffer solutions with ladder diagrams; test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. a solution. Buffer Solution Questions And Answers.
From www.chegg.com
Solved QUESTION 34 Buffers To answer this question • Write Buffer Solution Questions And Answers a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. Calculate the ph of an unbuffered 0.010m acetic acid solution. Here, we have a few questions with answers based on the buffer. how good are you at. Buffer Solution Questions And Answers.
From www.numerade.com
SOLVED III. Buffer solution Answer the following show you questions Buffer Solution Questions And Answers systematic solution to buffer problems; Calculate the ph of an unbuffered 0.010m acetic acid solution. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. test your knowledge on buffer solutions with these practice problems on. Buffer Solution Questions And Answers.
From www.chegg.com
Solved 5.Enter your data for the acetate buffer solutions A, Buffer Solution Questions And Answers Representing buffer solutions with ladder diagrams; Calculate the ph of an unbuffered 0.010m acetic acid solution. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. Here, we have a few questions with answers based on the buffer. the ability of a buffer solution to resist large changes in ph has. Buffer Solution Questions And Answers.
From www.studocu.com
Chapter 11 Buffers practice questions ( solutions) 636 pharmaceutics Buffer Solution Questions And Answers Calculate the ph of an unbuffered 0.010m acetic acid solution. how good are you at solving buffer solution practice problems? test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a. Buffer Solution Questions And Answers.
From criticalthinking.cloud
buffer solution questions a level chemistry Buffer Solution Questions And Answers Representing buffer solutions with ladder diagrams; Calculate the ph of an unbuffered 0.010m acetic acid solution. how good are you at solving buffer solution practice problems? Here, we have a few questions with answers based on the buffer. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. systematic solution. Buffer Solution Questions And Answers.
From www.chegg.com
Solved Part 4 Questions 1. a) What is a buffer solution? b) Buffer Solution Questions And Answers a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. Here, we have a few questions with. Buffer Solution Questions And Answers.
From www.youtube.com
Buffer Solution Questions Part2 IIT JEE NEET YouTube Buffer Solution Questions And Answers a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. systematic solution to buffer problems; the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. . Buffer Solution Questions And Answers.
From kunduz.com
[ANSWERED] Exercise N Identifying buffer solutions State whether each Buffer Solution Questions And Answers a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. how good are you at solving buffer solution practice. Buffer Solution Questions And Answers.
From www.numerade.com
SOLVED Your Name; and Buffers (7). Answer the following questions (a Buffer Solution Questions And Answers systematic solution to buffer problems; the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. . Buffer Solution Questions And Answers.
From kunduz.com
[ANSWERED] Which of the following is a buffer solution Question Type Buffer Solution Questions And Answers Representing buffer solutions with ladder diagrams; a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. . Buffer Solution Questions And Answers.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Questions And Answers how good are you at solving buffer solution practice problems? Calculate the ph of an unbuffered 0.010m acetic acid solution. Here, we have a few questions with answers based on the buffer. systematic solution to buffer problems; test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. Representing buffer solutions. Buffer Solution Questions And Answers.
From www.chegg.com
Solved A buffer solution contains 0.25 M NaNO2 and 0.75 M Buffer Solution Questions And Answers systematic solution to buffer problems; test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. Representing buffer solutions with ladder diagrams; Calculate the ph of an unbuffered 0.010m acetic acid solution. Here, we have a few questions with answers based on the buffer. how good are you at solving buffer. Buffer Solution Questions And Answers.
From studylib.net
Buffer Problems with answers Buffer Solution Questions And Answers systematic solution to buffer problems; how good are you at solving buffer solution practice problems? Representing buffer solutions with ladder diagrams; the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating. Buffer Solution Questions And Answers.
From www.studocu.com
Practice Question 3 Buffers Solution Buffers what happens when we add Buffer Solution Questions And Answers how good are you at solving buffer solution practice problems? test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. Here, we have a few questions with answers based on the buffer. Representing buffer solutions with ladder diagrams; the ability of a buffer solution to resist large changes in ph. Buffer Solution Questions And Answers.
From www.studypool.com
SOLUTION Acids bases and buffers mcq test with questions and answers Buffer Solution Questions And Answers Representing buffer solutions with ladder diagrams; systematic solution to buffer problems; how good are you at solving buffer solution practice problems? Here, we have a few questions with answers based on the buffer. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. Calculate the ph of. Buffer Solution Questions And Answers.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Solution Questions And Answers how good are you at solving buffer solution practice problems? Calculate the ph of an unbuffered 0.010m acetic acid solution. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. Representing buffer solutions with ladder diagrams; Here, we have a few questions with answers based on the buffer. a solution. Buffer Solution Questions And Answers.
From www.scribd.com
Worksheet Answers PDF Buffer Solution Acid Buffer Solution Questions And Answers a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. Representing buffer solutions with ladder diagrams; Calculate the ph of an unbuffered 0.010m acetic acid solution. Here, we have a few questions with answers based on the buffer.. Buffer Solution Questions And Answers.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Questions And Answers Representing buffer solutions with ladder diagrams; test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. the ability of. Buffer Solution Questions And Answers.
From www.numerade.com
SOLVED Explain Buffers and How They Work Question buffer solution made Buffer Solution Questions And Answers test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. Calculate the ph of an unbuffered 0.010m acetic acid solution.. Buffer Solution Questions And Answers.
From studylib.net
Chemguide answers BUFFER SOLUTIONS Buffer Solution Questions And Answers Here, we have a few questions with answers based on the buffer. systematic solution to buffer problems; test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. Representing buffer solutions with ladder diagrams; a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an. Buffer Solution Questions And Answers.
From studylib.net
Buffer Equilibrium Free Response Questions Buffer Solution Questions And Answers test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. systematic solution to buffer problems; how good are you at solving buffer solution practice problems? Representing buffer solutions with ladder diagrams; Here, we have a few questions with answers based on the buffer. the ability of a buffer solution. Buffer Solution Questions And Answers.
From www.chegg.com
Solved Buffer Lab Experiment please fill in the table on Buffer Solution Questions And Answers Here, we have a few questions with answers based on the buffer. test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. systematic solution to buffer problems; a solution. Buffer Solution Questions And Answers.
From www.tes.com
Buffer Solutions 3 (A Level Chemistry) Teaching Resources Buffer Solution Questions And Answers the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. Calculate the ph of an unbuffered 0.010m acetic acid solution. Here, we have a few questions with answers based on the buffer. systematic solution to buffer problems; a solution of acetic acid and sodium acetate (ch 3. Buffer Solution Questions And Answers.
From www.chegg.com
Solved Table 3 Sodium Acetate Data Table 4 Buffer Buffer Solution Questions And Answers how good are you at solving buffer solution practice problems? Calculate the ph of an unbuffered 0.010m acetic acid solution. Representing buffer solutions with ladder diagrams; the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. a solution of acetic acid and sodium acetate (ch 3 cooh. Buffer Solution Questions And Answers.
From www.formsbank.com
Buffer Solutions Worksheet With Answers printable pdf download Buffer Solution Questions And Answers test your knowledge on buffer solutions with these practice problems on identifying, preparing and calculating buffer solutions. the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. how good are you at solving buffer solution practice problems? Here, we have a few questions with answers based on. Buffer Solution Questions And Answers.
From www.youtube.com
Buffer solution Questions part1 NEET IIT JEE CSBE students YouTube Buffer Solution Questions And Answers Calculate the ph of an unbuffered 0.010m acetic acid solution. systematic solution to buffer problems; a solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its conjugate base,. the ability of a buffer solution to resist large changes in. Buffer Solution Questions And Answers.
From www.chegg.com
Solved it Buffer solution Answer the toflowing questions Buffer Solution Questions And Answers how good are you at solving buffer solution practice problems? Here, we have a few questions with answers based on the buffer. systematic solution to buffer problems; the ability of a buffer solution to resist large changes in ph has a great many chemical applications, but perhaps. Calculate the ph of an unbuffered 0.010m acetic acid solution.. Buffer Solution Questions And Answers.