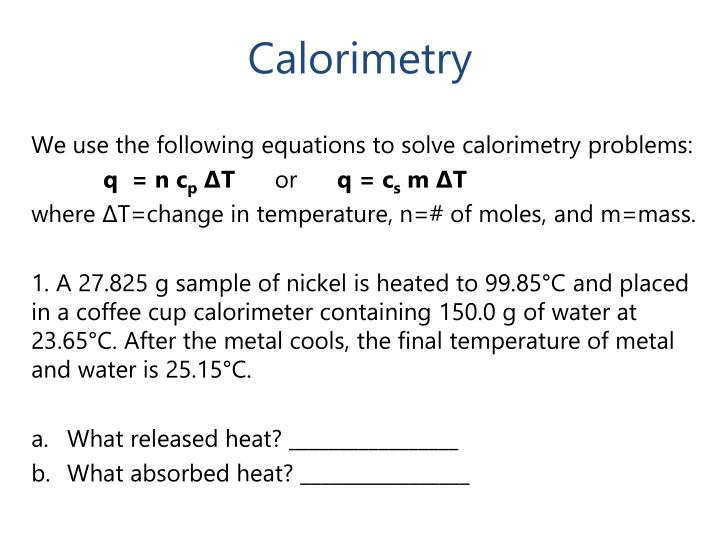
Calorimetry Equation Practice . How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. If the heat capacity of the. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! calorimetry practice problems 1. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,.
from www.slideserve.com
in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry practice problems 1. If the heat capacity of the. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat.
PPT Calorimetry PowerPoint Presentation ID6655927
Calorimetry Equation Practice in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry practice problems 1. If the heat capacity of the.
From www.gbu-presnenskij.ru
Chapter 25 Essential Calorimetry Formulae James Kennedy, 47 OFF Calorimetry Equation Practice this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? If the heat capacity of the. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the. Calorimetry Equation Practice.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Equation Practice calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. If the heat capacity of the. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by. Calorimetry Equation Practice.
From teachsimple.com
Calorimetry Practice by Teach Simple Calorimetry Equation Practice calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! If the heat capacity of the. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to. Calorimetry Equation Practice.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Equation Practice when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. If the heat capacity of the. . Calorimetry Equation Practice.
From www.youtube.com
How to find calorimeter constant YouTube Calorimetry Equation Practice calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. If the heat capacity of the. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate. Calorimetry Equation Practice.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe Calorimetry Equation Practice calorimetry practice problems 1. this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. calorimetry is used to measure the amount of thermal energy transferred in a chemical or. Calorimetry Equation Practice.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Equation Practice assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,.. Calorimetry Equation Practice.
From studydavid123.z13.web.core.windows.net
How To Calculate Calorimetry Problems Calorimetry Equation Practice calorimetry practice problems 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s. Calorimetry Equation Practice.
From www.scribd.com
Calorimetry Practice Worksheet PDF Calorimetry Equation Practice calorimetry practice problems 1. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. If the heat capacity of the. How much energy is needed to change the temperature of 50.0 g. Calorimetry Equation Practice.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Equation Practice calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? calorimetry practice problems 1. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat. Calorimetry Equation Practice.
From www.scribd.com
Bomb Calorimetry Practice Problems Calorimetry Equation Practice How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. calorimetry practice problems 1. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. a. Calorimetry Equation Practice.
From testbook.com
Principle of Calorimetry Definition, Formula, Uses, Examples Calorimetry Equation Practice a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. calorimetry practice problems. Calorimetry Equation Practice.
From dxohjtfln.blob.core.windows.net
Calorimetry Mass Formula at Thomas Despain blog Calorimetry Equation Practice calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. calorimetry practice problems 1.. Calorimetry Equation Practice.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Calorimetry Equation Practice If the heat capacity of the. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. assuming the specific heat of the solution and products is 4.20 j/g °c,. Calorimetry Equation Practice.
From www.numerade.com
SOLVEDThe defining equation for calorimetry is Calorimetry Equation Practice in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat.. Calorimetry Equation Practice.
From studylib.net
10.3 Calorimetry Practice Problems Name________________________________ H Calorimetry Equation Practice assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. If the heat capacity of the. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by. Calorimetry Equation Practice.
From worksheetmediadwaum.z14.web.core.windows.net
How To Calculate Calorimetry Problems Calorimetry Equation Practice When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48. Calorimetry Equation Practice.
From learningschoolconsoled.z14.web.core.windows.net
Coffee Cup Calorimeter Practice Problems Calorimetry Equation Practice assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. calorimetry practice. Calorimetry Equation Practice.
From www.youtube.com
Lecture 1.13 simple calorimetry problems YouTube Calorimetry Equation Practice this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. How much. Calorimetry Equation Practice.
From studylib.net
Calorimetry Worksheet Calorimetry Equation Practice When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. How. Calorimetry Equation Practice.
From classlibrarycordeiro.z21.web.core.windows.net
How To Calculate Calorimetry Problems Calorimetry Equation Practice a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. . Calorimetry Equation Practice.
From www.examples.com
Unit 6.3 Heat Capacity and Calorimetry (Notes & Practice Questions Calorimetry Equation Practice a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. calorimetry practice problems 1. When analysing. Calorimetry Equation Practice.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry Equation Practice this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. calorimetry practice problems. Calorimetry Equation Practice.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 Calorimetry Equation Practice If the heat capacity of the. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the. Calorimetry Equation Practice.
From www.studocu.com
Calorimetry Practice Problems a. How much energy is needed to raise Calorimetry Equation Practice when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. a collection of. Calorimetry Equation Practice.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Equation Practice How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. calorimetry is used to measure. Calorimetry Equation Practice.
From www.chegg.com
Solved Chapter 6 Calorimetry Worksheet Key Open the Calorimetry Equation Practice calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. If the heat capacity of the. calorimetry practice problems 1. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. when 1.00 g of coal is burned in a. Calorimetry Equation Practice.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Equation Practice assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. If the heat capacity of the. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. How much energy is needed to change the temperature of 50.0 g of. Calorimetry Equation Practice.
From studylib.net
Calorimetry practice Calorimetry Equation Practice assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. If the heat. Calorimetry Equation Practice.
From www.tessshebaylo.com
Equation For Constant Volume Calorimetry Tessshebaylo Calorimetry Equation Practice How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. calorimetry practice. Calorimetry Equation Practice.
From www.scribd.com
Calorimetry Practice Problems WS2 PDF Calorie Heat Calorimetry Equation Practice when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat. When analysing a heat. Calorimetry Equation Practice.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry Equation Practice When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass,. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical. Calorimetry Equation Practice.
From studylib.net
Calorimetry Problems Calorimetry Equation Practice in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. How. Calorimetry Equation Practice.
From studylib.net
Calorimetry Worksheet Calorimetry Equation Practice calorimetry practice problems 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? a collection of calorimetry exercises, all with solutions and steps solved in detail and accompanied by explanations! in this set of practice questions, we will go over the main types of questions on calorimetry including the heat. Calorimetry Equation Practice.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimetry Equation Practice in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. this quiz helps you practice calorimetry calculations, including heat exchanged and temperature change using a variety of materials. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the. Calorimetry Equation Practice.